电化学(中英文) ›› 2022, Vol. 28 ›› Issue (2): 2108431. doi: 10.13208/j.electrochem.210843
所属专题: “下一代二次电池”专题文章
王加义1,2, 郭胜楠1, 王新2,*( ), 谷林1, 苏东1,*(
), 谷林1, 苏东1,*( )
)
收稿日期:2021-09-13
修回日期:2021-10-28
出版日期:2022-02-28
发布日期:2021-11-02
Jia-Yi Wang1,2, Sheng-Nan Guo1, Xin Wang2,*( ), Lin Gu1, Dong Su1,*(
), Lin Gu1, Dong Su1,*( )
)
Received:2021-09-13
Revised:2021-10-28
Published:2022-02-28
Online:2021-11-02
Contact:
*Tel: (86-758)6635020, E-mail:
摘要:
高镍层状氧化物具有成本低、能量密度高的优点,被认为是新一代锂离子电池的理想正极材料。然而,由于在使用中其结构的耐久性与安全性问题,在实际应用过程中仍然面临着严峻的挑战。深入了解电极材料容量衰减过程中的结构演变对发展高性能层状氧化物电极材料具有重要的指导意义。本文综述了近年来高镍层状氧化物正极失效机理的研究进展,包括从高镍层状氧化物的内部结构演变、表面成分变化和热失控条件下的性质等方面,进行了详细的梳理。之后,本文介绍了国内外最新的高镍层状氧化物的改性策略,并对高镍氧化物正极结构研究的发展方向进行了总结和展望。
王加义, 郭胜楠, 王新, 谷林, 苏东. 锂离子电池高镍层状氧化物正极结构失效机制[J]. 电化学(中英文), 2022, 28(2): 2108431.
Jia-Yi Wang, Sheng-Nan Guo, Xin Wang, Lin Gu, Dong Su. Structural Degradation of Ni-Rich Layered Oxide Cathode for Li-Ion Batteries[J]. Journal of Electrochemistry, 2022, 28(2): 2108431.
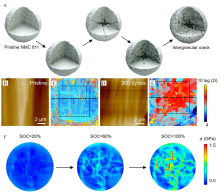
Figure 2
(A) The production of intergranular crack. Reproduced with permission of Ref.[46], copyright 2020 Elsevier. The (B, D) topographic and (C, E) corresponding Log(resistance) images at different stages of the sample. Reproduced with permission of Ref.[55], copyright 2018 Elsevier. (F) The stress distribution of NMC811 at different SOC values. Reproduced with permission of Ref.[56], copyright 2021 American Chemical Society. (color on line)

Figure 3
(A) Schematic diagram showing microcrack generation of NMC and (B) the corresponding SEM images of NMC with di-fferent Ni contents; Reproduced with permission of Ref.[59], copyright 2018 American Chemical Society. (C) SEM images of the cathode after different cycles. Reproduced with permission of Ref.[60], copyright 2013 Wiley-VCH. (color on line)

Figure 4
(A) In-situ XRD pattern of NMC811 cathode. Lattice changes of (B) a-axis and (C) c-axis as a function of voltage. Reproduced with permission of Ref.[37], copyright 2019 Wiley-VCH. (D) 6Li spin-echo ex-situ NMR spectra of NMC811; (E) the schematic diagram of phase transformation along the c-axis. The grey, green, pink and purple balls represent Li, Ni, Co and Mn atoms, respectively. Reproduced with permission of Ref.[61], copyright 2019 Elsevier. (color on line)
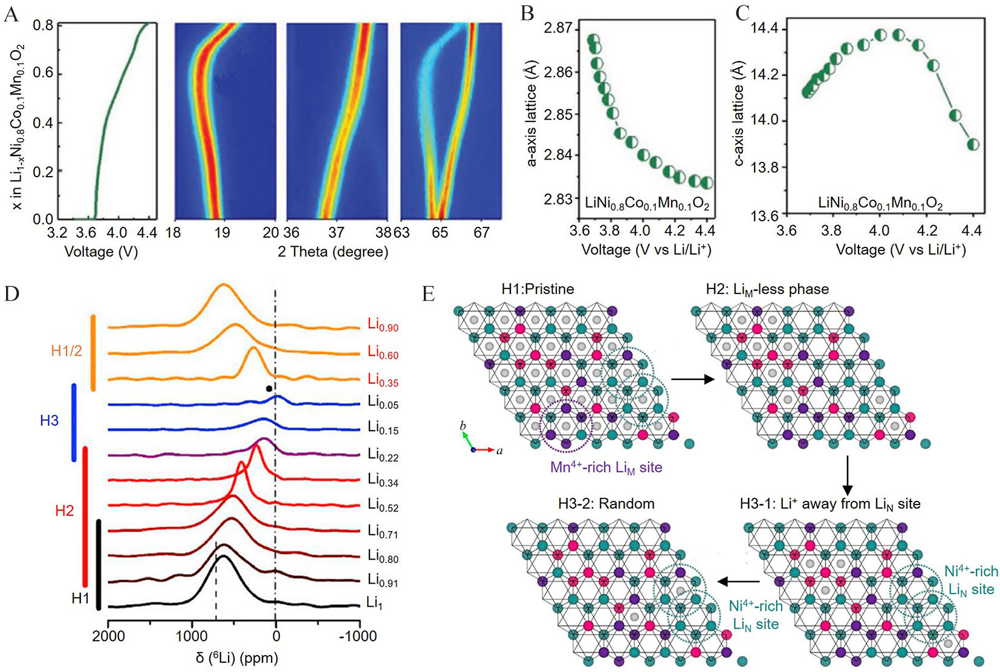
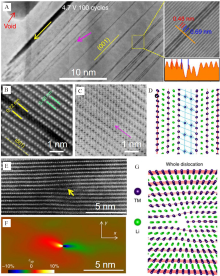
Figure 5
(A) STEM image of cycled NMC333 particles; (B) the atomic resolution STEM image and (C) the corresponding ABF image along [010] axis; (D) the corresponding schematic diagram; (E) the STEM image of NMC333 and (F) the corresponding strain map; (G) the corresponding schematic diagram. Reproduced with permission of Ref.[62], copyright 2017 Nature Publishing Group. (color on line)
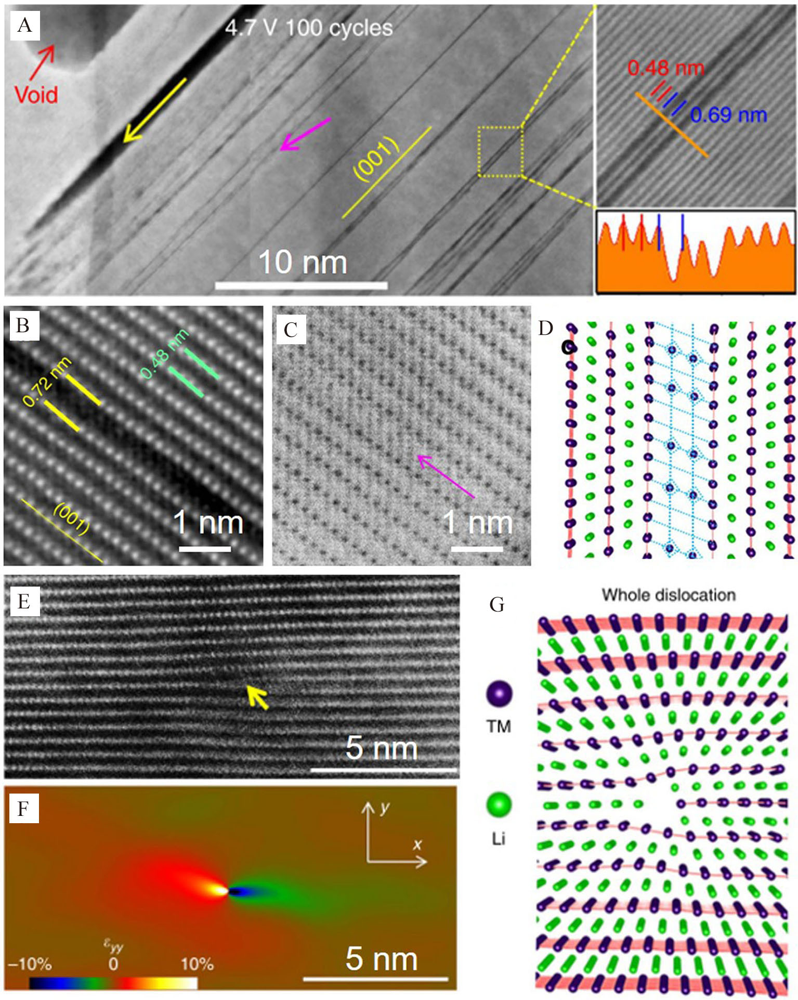
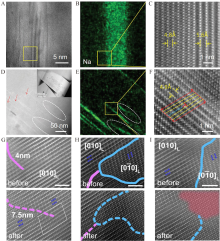
Figure 6
(A) The HAADF-STEM image, (B) Na element distribution of the selected area, (C) the atomic distribution of the selected area with L-type ion diffusion. (D) The HAADF image, (E) the Na element distribution, (F) the atomic distribution of the selected area with D-type ion diffusion. Reproduced with permission of Ref.[64], copyright 2019 Wiley-VCH. The STEM images with (G) pure APB, (H) APB and TB, and (I) TB before and after the delithiation. Reproduced with permission of Ref.[65], copyright 2020 Wiley-VCH. (color on line)
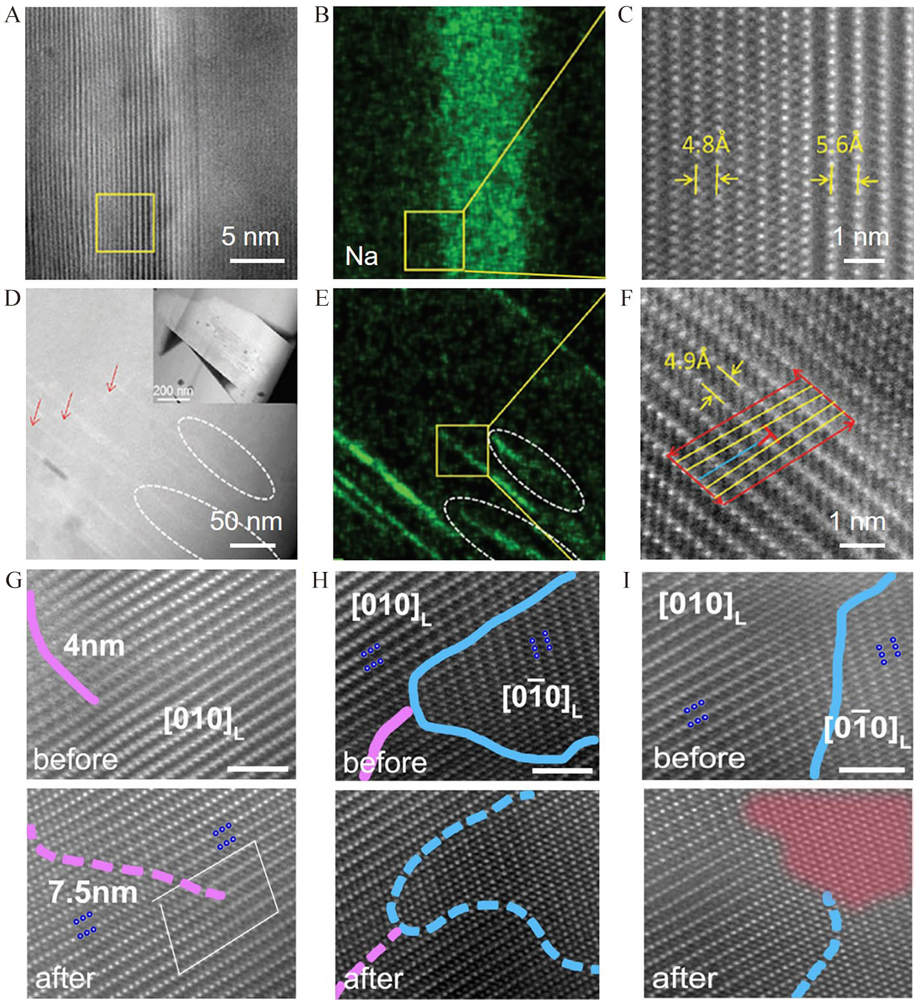
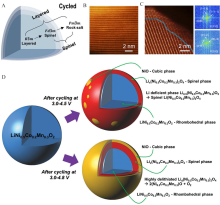
Figure 7
(A) Typical phase transformation schematic diagram. Reproduced with permission of Ref.[47], copyright 2019 Wiley-VCH. (B) The high-resolution STEM images of (B) pristine and (C) cycled NMCT particle as well as FFT patterns for the layered and reconstruction part. Reproduced with permission of Ref.[73], copyright 2014 Nature Publishing Group. (D) the structure evolution of NMC cathode under different voltages. Reproduced with permission of Ref.[74], copyright 2014 Wiley-VCH. (color on line)
Figure 8
STEM images of NMC811 after (A) 0 cycle, (B) 20 cycles, (C) 50 cycles and (D) 100 cycles. The STEM image of NMC811 at different regions including (E) layered phase; (F) disordered layered phase; (G) defect rock salt phase and (H) rock salt phase. Reproduced with permission of Ref.[76], copyright 2018 Elsevier. (I) the spacing ratio of the c direction and a direction; the lattice mismatch in the c and a directions; the schematic diagram showing structures of the (J) active NMC811 and (K) fatigued NMC811. Reproduced with permission of Ref.[79], copyright 2021 Nature Publishing Group. (color on line)
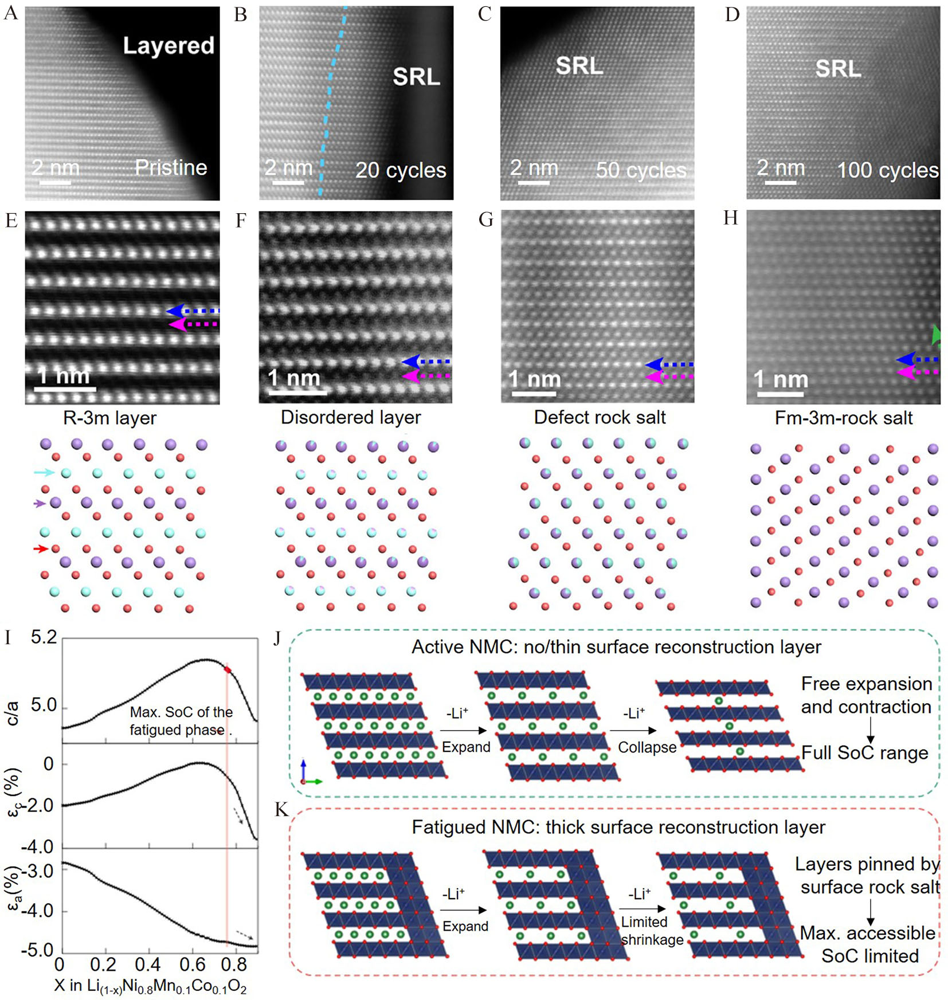
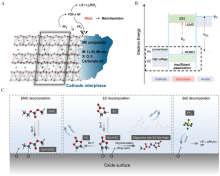
Figure 9
(A) The schematic diagram of the side reactions in the electrode-electrolyte interface. Reproduced with permission of Ref.[47], copyright 2019 Wiley-VCH. (B) the relation between anode/cathode working voltage and the energy level of electrolyte. Reproduced with permission of Ref.[80], copyright 2017 Royal Society of Chemistry. (C) the electrolyte decomposition mechanism on NMC811. Reproduced with permission of Ref.[81], copyright 2020 Royal Society of Chemistry. (color on line)
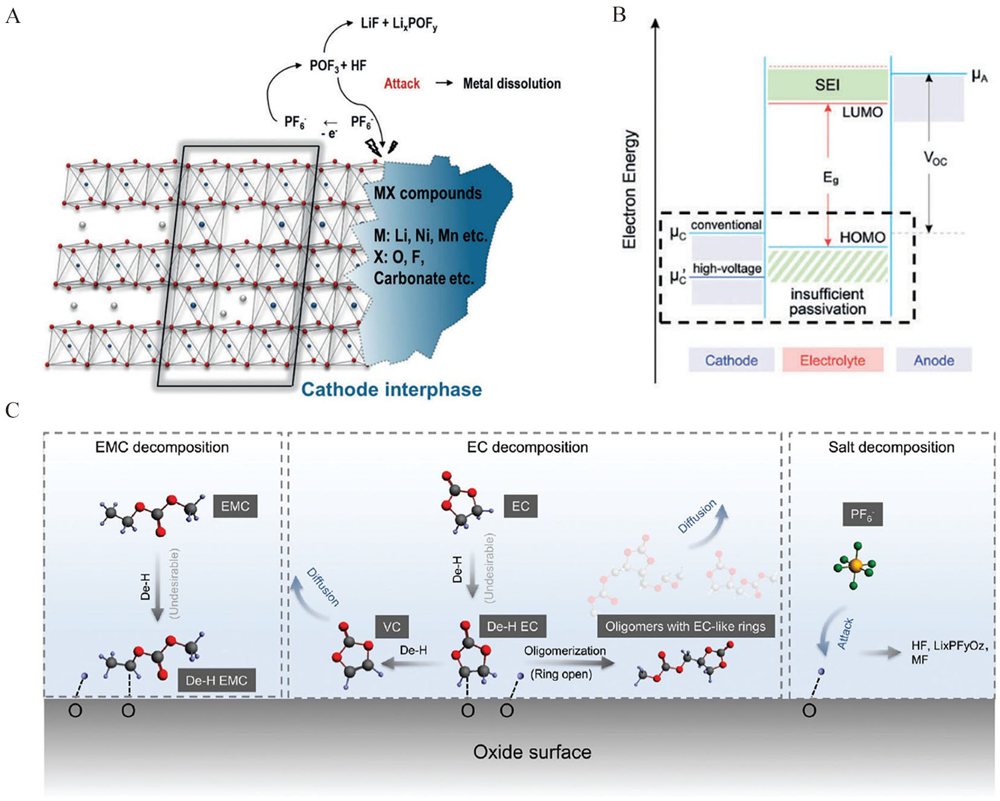
Figure 10
(A) the relationship of thermal stability, capacity and capacity retention. Reproduced with permission of Ref.[86], copyright 2013 Elsevier. (B) TR-XRD/MS patterns. Reproduced with permission of Ref.[87], copyright 2013 American Chemical Society. (C-E) the cathode component illustration and the possible reaction mechanisms. Reproduced with permission of Ref.[88], copyright 2021 Elsevier. (color on line)


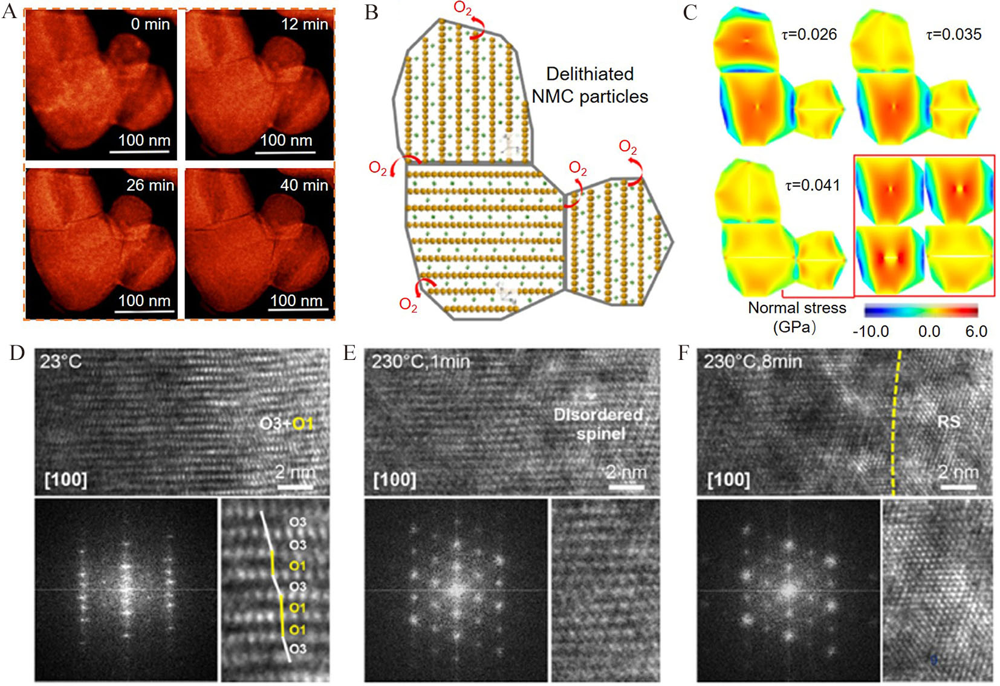
Figure 11
(A) STEM images of NMC442 at 230 °C for 0 min, 12 min, 26 min and 40 min. (B) the constructed FEM model for NMC442 particle. (C) structure evolution of NMC442 particle caused by the oxygen release. Reproduced with permission of Ref.[90], copyright 2018 American Chemical Society. (D-F) the HRTEM images of LiNiO2 at 230 °C for 0 min, 1 min and 8 min. Reproduced with permission of Ref.[91], copyright 2021 Cell Press. (color on line)
| [1] |
Jung S K, Hwang I, Chang D, Park K Y, Kim S J, Seong W M, Eum D, Park J, Kim B, Kim J, Heo J H, Kang K. Nanoscale phenomena in lithium-ion batteries[J]. Chem. Rev., 2020, 120(14):6684-6737.
doi: 10.1021/acs.chemrev.9b00405 URL |
| [2] |
Xue W, Huang M, Li Y, Zhu Y G, Gao R, Xiao X, Zhang W, Li S, Xu G, Yu Y, Li P, Lopez J, Yu D, Dong Y, Fan W, Shi Z, Xiong R, Sun C J, Hwang I, Lee W K, Shao H Y, Johnson J A, Li J. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte[J]. Nat. Energy, 2021, 6:495-505.
doi: 10.1038/s41560-021-00792-y URL |
| [3] |
Manthiram A. A reflection on lithium-ion battery cathode chemistry[J]. Nat. Commun., 2020, 11(1):1550.
doi: 10.1038/s41467-020-15355-0 pmid: 32214093 |
| [4] |
Or T, Gourley S W D, Kaliyappan K, Yu A, Chen Z W. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook[J]. Carbon Energy, 2020, 2(1):6-43.
doi: 10.1002/cl2.v2.1 URL |
| [5] |
Liu Y, Zhai Y P, Xia Y Y, Li W, Zhao D Y. Recent progress of porous materials in lithium-metal batteries[J]. Small Structures, 2021, 2(5):2000118.
doi: 10.1002/sstr.v2.5 URL |
| [6] |
Li Y, Wu F, Qian J, Zhang M H, Yuan Y X, Bai Y, Wu C. Metal chalcogenides with heterostructures for high-performance rechargeable batteries[J]. Small Science, 2021, 1(9):2100012.
doi: 10.1002/smsc.v1.9 URL |
| [7] |
Gong D C, Wei C Y, Liang Z W, Tang Y B. Recent advances on sodium-ion batteries and sodium dual-ion batteries: State-of-the-art Na+ host anode materials[J]. Small Science, 2021, 1(6):2100014.
doi: 10.1002/smsc.v1.6 URL |
| [8] |
Zuo W H, Luo M Z, Liu X S, Wu J, Liu H D, Li J, Winter M, Fu R Q, Yang W L, Yang Y. Li-rich cathodes for rechargeable Li-based batteries: Reaction mechanisms and advanced characterization techniques[J]. Energ. Environ. Sci., 2020, 13(12):4450-4497.
doi: 10.1039/D0EE01694B URL |
| [9] |
Qiao Y, Yang H J, Chang Z, Deng H, Li X, Zhou H S. A high-energy-density and long-life initial-anode-free lithium battery enabled by a Li2O sacrificial agent[J]. Nat. Energy, 2021, 6(6):653-662.
doi: 10.1038/s41560-021-00839-0 URL |
| [10] |
Zheng J X, Ye Y K, Liu T C, Xiao Y G, Wang C M, Wang F, Pan F. Ni/Li disordering in layered transition metal oxide: Electrochemical impact, origin, and control[J]. Accounts Chem. Res., 2019, 52(8):2201-2209.
doi: 10.1021/acs.accounts.9b00033 URL |
| [11] |
Wu J X, Cao Y L, Zhao H M, Mao J F, Guo Z P. The critical role of carbon in marrying silicon and graphite anodes for high-energy lithium-ion batteries[J]. Carbon Energy, 2019, 1(1):57-76.
doi: 10.1002/cl2.v1.1 URL |
| [12] |
Yu L, Wang J, Xu Z J. A perspective on the behavior of lithium anodes under a magnetic field[J]. Small Structures, 2020, 2(1):2000043.
doi: 10.1002/sstr.v2.1 URL |
| [13] | Song Y W, Peng Y Q, Zhao M, Lu Y, Liu J N, Li B Q, Zhang Q. Understanding the impedance response of lithium polysulfide symmetric cells[J]. Small Science, 2021: 2100042. |
| [14] | Zhao S Q, Guo Z Q, Yan K, Wan S W, He F R, Sun B, Wang G X. Towards high-energy-density lithium-ion batteries: Strategies for developing high-capacity lithium-rich cathode materials[J]. Energy Storage Mater., 2021, 34:716-734. |
| [15] |
Li T Y, Yuan X Z, Zhang L, Song D T, Shi K Y, Bock C. Degradation mechanisms and mitigation strategies of nickel-rich NMC-based lithium-ion batteries[J]. Electrochem. Energy Rev., 2019, 3(1):43-80.
doi: 10.1007/s41918-019-00053-3 URL |
| [16] |
Li J, Hwang S, Guo F M, Li S, Chen Z W, Kou R H, Sun K, Sun C J, Gan H, Yu A P, Stach E A, Zhou H, Su D. Phase evolution of conversion-type electrode for lithium ion batteries[J]. Nat. Commun., 2019, 10(1):2224.
doi: 10.1038/s41467-019-09931-2 URL |
| [17] |
Ren J C, Huang Y L, Zhu H, Zhang B H, Zhu H K, Shen S H, Tan G Q, Wu F, He H, Lan S, Xia X H, Liu Q. Recent progress on MOF-derived carbon materials for energy storage[J]. Carbon Energy, 2020, 2(2):176-202.
doi: 10.1002/cl2.v2.2 URL |
| [18] |
Zhang X D, Yue F S, Liang J Y, Shi J L, Li H, Guo Y G. Structure design of cathode electrodes for solid-state batteries: Challenges and progress[J]. Small Structures, 2020, 1(3):2000042.
doi: 10.1002/sstr.v1.3 URL |
| [19] |
Meng X Y, Sun Y F, Yu M Z, Wang Z Y, Qiu J S. Hydrogen-bonding crosslinking mxene to highly robust and ultralight aerogels for strengthening lithium metal anode[J]. Small Science, 2021, 1(9):2100021.
doi: 10.1002/smsc.v1.9 URL |
| [20] |
Hou P Y, Yin J M, Ding M, Huang J Z, Xu X J. Surface/interfacial structure and chemistry of high-energy nickel-rich layered oxide cathodes: Advances and perspectives[J]. Small, 2017, 13(45):1701802.
doi: 10.1002/smll.v13.45 URL |
| [21] |
Duffner F, Kronemeyer N, Tübke J, Leker J, Winter M, Schmuch R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure[J]. Nat. Energy, 2021, 6(2):123-134.
doi: 10.1038/s41560-020-00748-8 URL |
| [22] |
Cai W, Yan C, Yao Y X, Xu L, Xu R, Jiang L L, Huang J Q, Zhang Q. Rapid lithium diffusion in order@disorder pathways for fast-charging graphite anodes[J]. Small Structures, 2020, 1(1):2000010.
doi: 10.1002/sstr.v1.1 URL |
| [23] |
Chen L, Su Y F, Chen S, Li N, Bao L Y, Li W K, Wang Z, Wang M, Wu F. Hierarchical Li1.2Ni0.2Mn0.6O2 nanoplates with exposed {010} planes as high-performance cathode material for lithium-ion batteries[J]. Adv. Mater., 2014, 26(39):6756-6760.
doi: 10.1002/adma.v26.39 URL |
| [24] |
Lee S Y, Park G S, Jung C, Ko D S, Park S Y, Kim H G, Hong S H, Zhu Y, Kim M. Revisiting primary particles in layered lithium transition-metal oxides and their impact on structural degradation[J]. Adv. Sci., 2019, 6(6):1800843.
doi: 10.1002/advs.v6.6 URL |
| [25] |
Nitta N, Wu F, Lee J T, Yushin G. Li-ion battery materials: Present and future[J]. Mater. Today, 2015, 18(5):252-264.
doi: 10.1016/j.mattod.2014.10.040 URL |
| [26] |
Bhuvaneswari S, Varadaraju U V, Gopalan R, Prakash R. Structural stability and superior electrochemical performance of Sc-doped LiMn2O4 spinel as cathode for lithium ion batteries[J]. Electrochim. Acta, 2019, 301:342-351.
doi: 10.1016/j.electacta.2019.01.174 |
| [27] |
Galceran M, Guerfi A, Armand M, Zaghib K, Casas C M. The critical role of carbon in the chemical delithiation kinetics of LiFePO4[J]. J. Electrochem. Soc., 2020, 167(7):070538.
doi: 10.1149/1945-7111/ab7ce3 URL |
| [28] |
Alsamet M A M M, Burgaz E. Synjournal and characterization of nano-sized LiFePO4 by using consecutive combination of sol-gel and hydrothermal methods[J]. Electrochim. Acta, 2021, 367:137530.
doi: 10.1016/j.electacta.2020.137530 URL |
| [29] |
Bai Y, Li L M, Li Y, Chen G H, Zhao H C, Wang Z H, Wu C, Ma H Y, Wang X Q, Cui H Y, Zhou J. Reversible and irreversible heat generation of NCA/Si-C pouch cell during electrochemical energy-storage process[J]. J. Energy Chem., 2019, 29:95-102.
doi: 10.1016/j.jechem.2018.02.016 URL |
| [30] |
Xia S B, Huang W J, Shen X, Liu J M, Cheng F X, Liu J J, Yang X F, Guo H. Rearrangement on surface structures by boride to enhanced cycle stability for LiNi0.80Co0.15Al0.05O2 cathode in lithium ion batteries[J]. J. Energy Chem., 2020, 45:110-118.
doi: 10.1016/j.jechem.2019.09.023 URL |
| [31] |
Kim J, Cho H, Jeong H Y, Ma H, Lee J, Hwang J, Park M, Cho J. Self-induced concentration gradient in nickel-rich cathodes by sacrificial polymeric bead clusters for high-energy lithium-ion batteries[J]. Adv. Energy Mater., 2017, 7(12):1602559.
doi: 10.1002/aenm.v7.12 URL |
| [32] |
Li H, Zhou P F, Liu F M, Li H X, Cheng F Y, Chen J. Stabilizing nickel-rich layered oxide cathodes by magnesium doping for rechargeable lithium-ion batteries[J]. Chem. Sci., 2019, 10(5):1374-1379.
doi: 10.1039/C8SC03385D URL |
| [33] |
Liang C P, Kong F T, Longo R C, Zhang C X, Nie Y F, Zheng Y P, Cho K. Site-dependent multicomponent doping strategy for Ni-rich LiNi1-2yCoyMnyO2 (y = 1/12) cathode materials for Li-ion batteries[J]. J. Mater. Chem. A, 2017, 5(48):25303-25313.
doi: 10.1039/C7TA08618K URL |
| [34] |
Li W, Erickson E M, Manthiram A. High-nickel layered oxide cathodes for lithium-based automotive batteries[J]. Nat. Energy, 2020, 5(1):26-34.
doi: 10.1038/s41560-019-0513-0 URL |
| [35] |
Kim U H, Kim J H, Hwang J Y, Ryu H H, Yoon C S, Sun Y K. Compositionally and structurally redesigned high-energy Ni-rich layered cathode for next-generation lithium batteries[J]. Mater. Today, 2019, 23:26-36.
doi: 10.1016/j.mattod.2018.12.004 URL |
| [36] |
Sun H H, Ryu H H, Kim U H, Weeks J A, Heller A, Sun Y K, Mullins C B. Beyond doping and coating: Prospective strategies for stable high-capacity layered Ni-rich cathodes[J]. ACS Energy Lett., 2020, 5(4):1136-1146.
doi: 10.1021/acsenergylett.0c00191 URL |
| [37] |
Li J Y, Manthiram A. A comprehensive analysis of the interphasial and structural evolution over long-term cycling of ultrahigh-nickel cathodes in lithium-ion batteries[J]. Adv. Energy Mater., 2019, 9(45):1902731.
doi: 10.1002/aenm.v9.45 URL |
| [38] |
Liu W, Oh P, Liu X, Lee M J, Cho W, Chae S, Kim Y, Cho J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries[J]. Angew. Chem. Int. Edit., 2015, 54(15):4440-4457.
doi: 10.1002/anie.201409262 URL |
| [39] |
Li H Y, Liu A R, Zhang N, Wang Y Q, Yin S, Wu H H, Dahn J R. An unavoidable challenge for Ni-rich positive electrode materials for lithium-ion batteries[J]. Chem. Mater., 2019, 31(18):7574-7583.
doi: 10.1021/acs.chemmater.9b02372 URL |
| [40] |
Li Y, Li X H, Wang Z X, Guo H J, Wang J X. Spray pyrolysis synjournal of nickel-rich layered cathodes LiNi1-2xCox-MnxO2 (x = 0.075, 0.05, 0.025) for lithium-ion batteries[J]. J. Energy Chem., 2018, 27(2):447-450.
doi: 10.1016/j.jechem.2017.11.025 URL |
| [41] |
Liu Y, Tang L B, Wei H X, Zhang X H, He Z J, Li Y J, Zheng J C. Enhancement on structural stability of Ni-rich cathode materials by in-situ fabricating dual-modified layer for lithium-ion batteries[J]. Nano Energy, 2019, 65:104043.
doi: 10.1016/j.nanoen.2019.104043 URL |
| [42] |
Kim U H, Ryu H H, Kim J H, Mücke R, Kaghazchi P, Yoon C S, Sun Y K. Microstructure-controlled Ni-rich cathode material by microscale compositional partition for next-generation electric vehicles[J]. Adv. Energy Mater., 2019, 9(15):1803902.
doi: 10.1002/aenm.v9.15 URL |
| [43] |
Zhang L Q, Zhu C X, Yu S C, Ge D H, Zhou H S. Status and challenges facing representative anode materials for rechargeable lithium batteries[J]. J. Energy Chem., 2022, 66:260-294.
doi: 10.1016/j.jechem.2021.08.001 URL |
| [44] |
Liang L W, Zhang W H, Zhao F, Denis D K, Zaman F U, Hou L R, Yuan C Z. Surface/interface structure degradation of Ni-rich layered oxide cathodes toward lithium-ion batteries: Fundamental mechanisms and remedying strategies[J]. Adv. Mater. Inter., 2019, 7(3):1901749.
doi: 10.1002/admi.v7.3 URL |
| [45] |
Hu D Z, Su Y F, Chen L, Li N, Bao L Y, Lu Y, Zhang Q Y, Wang J, Chen S, Wu F. The mechanism of side reaction induced capacity fading of Ni-rich cathode materials for lithium ion batteries[J]. J. Energy Chem., 2021, 58:1-8.
doi: 10.1016/j.jechem.2020.09.031 URL |
| [46] |
Lin Q Y, Guan W H, Zhou J B, Meng J, Huang W, Chen T, Gao Q, Wei X, Zeng Y W, Li J X, Zhang Z. Ni-Li anti-site defect induced intragranular cracking in Ni-rich layer-structured cathode[J]. Nano Energy, 2020, 76:105021.
doi: 10.1016/j.nanoen.2020.105021 URL |
| [47] |
Lee W, Muhammad S, Sergey C, Lee H, Yoon J, Kang Y M, Yoon W S. Advances in the cathode materials for lithium rechargeable batteries[J]. Angew. Chem. Int. Ed., 2020, 59(7):2578-2605.
doi: 10.1002/anie.v59.7 URL |
| [48] |
Nam K W, Bak S M, Hu E Y, Yu X Q, Zhou Y N, Wang X, Wu L, Zhu Y, Chung K Y, Yang X Q. Combining in situ synchrotron X-ray diffraction and absorption techniques with transmission electron microscopy to study the origin of thermal instability in overcharged cathode materials for lithium-ion batteries[J]. Adv. Funct. Mater., 2013, 23(8):1047-1063.
doi: 10.1002/adfm.v23.8 URL |
| [49] |
Yin S Y, Deng W T, Chen J, Gao X, Zou G Q, Hou H S, Ji X B. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries[J]. Nano Energy, 2021, 83:105854.
doi: 10.1016/j.nanoen.2021.105854 URL |
| [50] |
Qian G N, Zhang J, Chu S Q, Li J Z, Zhang K, Yuan Q X, Ma Z F, Pianetta P, Li L S, Jung K, Liu Y J. Understanding the mesoscale degradation in nickel-rich cathode materials through machine-learning-revealed strain-redox decoupling[J]. ACS Energy Lett., 2021, 6(2):687-693.
doi: 10.1021/acsenergylett.0c02699 URL |
| [51] | Tang Z F, Wang S, Liao J Y, Wang S, He X D, Pan B C, He H Y, Chen C H. Facilitating lithium-ion diffusion in layered cathode materials by introducing Li+/Ni2+ antisite defects for high-rate Li-ion batteries[J]. Research, 2019: UNSP2198906. |
| [52] |
Xu Z R, Jiang Z R, Kuai C G, Xu R, Qin C D, Zhang Y, Rahman M M, Wei C X, Nordlund D, Sun C J, Xiao X H, Du X W, Zhao K J, Yan P F, Liu Y J, Lin F. Charge distribution guided by grain crystallographic orientations in polycrystalline battery materials[J]. Nat. Commun., 2020, 11(1):83.
doi: 10.1038/s41467-019-13884-x URL |
| [53] |
Su Y F, Zhang Q Y, Chen L, Bao L Y, Lu Y, Chen S, Wu F. Stress accumulation in Ni-rich layered oxide cathodes: Origin, impact, and resolution[J]. J. Energy Chem., 2022, 65:236-253.
doi: 10.1016/j.jechem.2021.05.048 URL |
| [54] |
Yoon C S, Ryu H H, Park G T, Kim J H, Kim K H, Sun Y K. Extracting maximum capacity from Ni-rich Li[Ni0.95Co0.025Mn0.025]O2 cathodes for high-energy-density lithium-ion batteries[J]. J. Mater. Chem. A, 2018, 6(9):4126-4132.
doi: 10.1039/C7TA11346C URL |
| [55] |
Park S Y, Baek W J, Lee S Y, Seo J A, Kang Y S, Koh M, Kim S H. Probing electrical degradation of cathode materials for lithium-ion batteries with nanoscale resolution[J]. Nano Energy, 2018, 49:1-6.
doi: 10.1016/j.nanoen.2018.04.005 URL |
| [56] |
Cheng X P, Li Y H, Cao T C, Wu R, Wang M M, Liu H, Liu X Q, Lu J X, Zhang Y F. Real-time observation of chemomechanical breakdown in a layered nickel-rich oxide cathode realized by in situ scanning electron microscopy[J]. ACS Energy Lett., 2021, 6(5):1703-1710.
doi: 10.1021/acsenergylett.1c00279 URL |
| [57] |
Wu H Q, Qin C D, Wang K, Han X, Sui M L, Yan P F. Revealing two distinctive intergranular cracking mechanisms of Ni-rich layered cathode by cross-sectional scanning electron microscopy[J]. J. Power Sources, 2021, 503:230066.
doi: 10.1016/j.jpowsour.2021.230066 URL |
| [58] |
Xu Z R, Rahman M M, Mu L Q, Liu Y J, Lin F. Chemomechanical behaviors of layered cathode materials in alkali metal ion batteries[J]. J. Mater. Chem. A, 2018, 6(44):21859-21884.
doi: 10.1039/C8TA06875E URL |
| [59] |
Ryu H H, Park K J, Yoon C S, Sun Y K. Capacity fading of Ni-rich Li[NixCoyMn1-x-y]O2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation?[J]. Chem. Mater., 2018, 30(3):1155-1163.
doi: 10.1021/acs.chemmater.7b05269 URL |
| [60] |
Miller D J, Proff C, Wen J G, Abraham D P, Bareño J. Observation of microstructural evolution in Li battery cathode oxide particles by in situ electron microscopy[J]. Adv. Energy Mater., 2013, 3(8):1098-1103.
doi: 10.1002/aenm.v3.8 URL |
| [61] |
Zheng S Y, Hong C Y, Guan X Y, Xiang Y X, Liu X S, Xu G L, Liu R, Zhong G M, Zheng F, Li Y X, Zhang X Y, Ren Y, Chen Z H, Amine K, Yang Y. Correlation between long range and local structural changes in Ni-rich layered materials during charge and discharge process[J]. J. Power Sources, 2019, 412:336-343.
doi: 10.1016/j.jpowsour.2018.11.053 URL |
| [62] |
Yan P F, Zheng J M, Gu M, Xiao J, Zhang J G, Wang C M. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries[J]. Nat. Commun., 2017, 8; 14101.
doi: 10.1038/ncomms14101 URL |
| [63] |
Zhang H L, Omenya F, Yan P F, Luo L L, Whittingham M S, Wang C M, Zhou G W. Rock-salt growth-induced (003) cracking in a layered positive electrode for Li-ion batteries[J]. ACS Energy Lett., 2017, 2(11):2607-2615.
doi: 10.1021/acsenergylett.7b00907 URL |
| [64] |
Xiao B W, Wang K, Xu G L, Song J H, Chen Z H, Amine K, Reed D, Sui M L, Sprenkle V, Ren Y, Yan P F, Li X L. Revealing the atomic origin of heterogeneous Li-ion diffusion by probing Na[J]. Adv. Mater., 2019, 31(29):1805889.
doi: 10.1002/adma.v31.29 URL |
| [65] |
Li S, Yao Z P, Zheng J M, Fu M S, Cen J J, Hwang S, Jin H L, Orlov A, Gu L, Wang S, Chen Z W, Su D. Direct observation of defect-aided structural evolution in a nickel-rich layered cathode[J]. Angew. Chem. Int. Ed., 2020, 59(49):22092-22099.
doi: 10.1002/anie.v59.49 URL |
| [66] | Qian G N, Zhang Y T, Li L S, Zhang R X, Xu J M, Cheng Z J, Xie S J, Wang H, Rao Q L, He Y S, Shen Y B, Chen L W, Tang M, Ma Z F. Single-crystal nickel-rich layered-oxide battery cathode materials: Synjournal, electrochemistry, and intra-granular fracture[J]. Energy Storage Mater., 2020, 27:140-149. |
| [67] |
Trevisanello E, Ruess R, Conforto G, Richter F H, Janek J. Polycrystalline and single crystalline ncm cathode materials-quantifying particle cracking, active surface area, and lithium diffusion[J]. Adv. Energy Mater., 2021, 11(18):2003400.
doi: 10.1002/aenm.v11.18 URL |
| [68] |
Yan P F, Zheng J M, Liu J, Wang B Q, Cheng X P, Zhang Y F, Sun X L, Wang C M, Zhang J G. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries[J]. Nat. Energy, 2018, 3(7):600-605.
doi: 10.1038/s41560-018-0191-3 URL |
| [69] |
Li Y W, Li Z B, Chen C, Yang K, Cao B, Xu S Y, Yang N, Zhao W G, Chen H B, Zhang M J, Pan F. Recent progress in Li and Mn rich layered oxide cathodes for Li-ion batteries[J]. J. Energy Chem., 2021, 61:368-385.
doi: 10.1016/j.jechem.2021.01.034 URL |
| [70] |
Shao M C, Shang C S, Zhang F X, Xu Z, Hu W, Lu Q Q, Gai L G. Selective adsorption-involved formation of NMC532/PANI microparticles with high ageing resistance and improved electrochemical performance[J]. J. Energy Chem., 2021, 54:668-679.
doi: 10.1016/j.jechem.2020.07.001 URL |
| [71] |
Qiu Q Q, Yuan S S, Bao J, Wang Q C, Yue X Y, Li X L, Wu X J, Zhou Y N. Suppressing irreversible phase transition and enhancing electrochemical performance of Ni-rich layered cathode LiNi0.9Co0.05Mn0.05O2 by fluorine substitution[J]. J. Energy Chem., 2021, 61:574-581.
doi: 10.1016/j.jechem.2021.02.012 URL |
| [72] |
Wu F, Liu N, Chen L, Li N, Dong J Y, Lu Y, Tan G Q, Xu M Z, Cao D Y, Liu Y F, Chen Y B, Su Y F. The nature of irreversible phase transformation propagation in nickel-rich layered cathode for lithium-ion batteries[J]. J. Energy Chem., 2021, 62:351-358.
doi: 10.1016/j.jechem.2021.03.035 URL |
| [73] |
Lin F, Markus I M, Nordlund D, Weng T C, Asta M D, Xin H L, Doeff M M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries[J]. Nat. Commun., 2014, 5:3529.
doi: 10.1038/ncomms4529 URL |
| [74] |
Jung S K, Gwon H, Hong J, Park K Y, Seo D H, Kim H, Hyun J, Yang W, Kang K. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries[J]. Adv. Energy Mater., 2014, 4(1):1300787.
doi: 10.1002/aenm.201300787 URL |
| [75] |
Tsutomu O, Atsushi U, Nagayama M. Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells[J]. J. Electrochem. Soc., 1993, 140(7):1862-1870.
doi: 10.1149/1.2220730 URL |
| [76] |
Lin Q Y, Guan W H, Meng J, Huang W, Wei X, Zeng Y W, Li J X, Zhang Z. A new insight into continuous performance decay mechanism of Ni-rich layered oxide cathode for high energy lithium ion batteries[J]. Nano Energy, 2018, 54:313-321.
doi: 10.1016/j.nanoen.2018.09.066 URL |
| [77] |
Wang J, Lu X, Zhang Y, Zhou J, Wang J, Xu S. A new insight into continuous performance decay mechanism of Ni-rich layered oxide cathode for high energy lithium ion batteries[J]. Nano Energy, 2018, 54:313-321.
doi: 10.1016/j.nanoen.2018.09.066 URL |
| [78] |
Zhang S S. Understanding of performance degradation of LiNi0.80Co0.10Mn0.10O2 cathode material operating at high potentials[J]. J. Energy Chem., 2020, 41:135-141.
doi: 10.1016/j.jechem.2019.05.013 URL |
| [79] |
Xu C, Marker K, Lee J, Mahadevegowda A, Reeves P J, Day S J, Groh M F, Emge S P, Ducati C, Layla Mehdi B, Tang C C, Grey C P. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries[J]. Nat. Mater., 2021, 20(1):84-92.
doi: 10.1038/s41563-020-0767-8 URL |
| [80] | Li W D, Song B H, Manthiram A. High-voltage positive electrode materials for lithium-ion batteries[J]. Chem. Soc. Rev., 2017, 46(10):3006-3059. |
| [81] | Zhang Y, Katayama Y, Tatara R, Giordano L, Yu Y, Fraggedakis D, Sun J G, Maglia F, Jung R, Bazant M Z, Shao H Y. Revealing electrolyte oxidation via carbonate dehydrogenation on Ni-based oxides in Li-ion batteries by in situ fourier transform infrared spectroscopy[J]. Energ Environ. Sci., 2020, 13(1):183-199. |
| [82] | Giordano L, Karayaylali P, Yu Y, Katayama Y, Maglia F, Lux S, Shao H Y. Chemical reactivity descriptor for the oxide-electrolyte interface in Li-ion batteries[J]. J. Phys. Chem. Lett., 2017, 8(16):3881-3887. |
| [83] | Zheng J X, Liu T C, Hu Z X, Wei Y, Song X H, Ren Y, Wang W D, Rao M M, Lin Y, Chen Z H, Lu J, Wang C M, Amine K, Pan F. Tuning of thermal stability in layered Li(NixMnyCoz)O2[J]. J. Am. Chem. Soc., 2016, 138(40):13326-13334. |
| [84] | Lin Y, Zhou M, Tai X L, Li H F, Han X, Yu J G. Analytical transmission electron microscopy for emerging advanced materials[J]. Matter, 2021, 4(7):2309-2339. |
| [85] | Zhang S C(张世超), Shen Z Y(沈泽宇), Lu Y Y(陆盈盈). Research progress of thermal runaway and safety for lithium metal batteries[J]. Acta Phys. -Chim. Sin.(物理化学学报), 2020, 37:2008065. |
| [86] | Noh H J, Youn S, Yoon C S, Sun Y K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries[J]. J. Power Sources, 2013, 233:121-130. |
| [87] | Bak S M, Nam K W, Chang W, Yu X, Hu E, Hwang S, Stach E A, Kim K B, Chung K Y, Yang X Q. Correlating structural changes and gas evolution during the thermal decomposition of charged LixNi0.8Co0.15Al0.05O2 cathode materials[J]. Chem. Mater., 2013, 25(3):337-351. |
| [88] | Li Y, Liu X, Wang L, Feng X N, Ren D S, Wu Y, Xu G L, Lu L G, Hou J X, Zhang W F, Wang Y L, Xu W Q, Ren Y, Wang Z F, Huang J Y, Meng X F, Han X B, Wang H W, He X M, Chen Z H, Amine K, Ouyang M G. Thermal runaway mechanism of lithium-ion battery with LiNi0.8Mn0.1Co0.1O2 cathode materials[J]. Nano Energy, 2021, 85:105878. |
| [89] | Alvarado J, Wei C X, Nordlund D, Kroll T, Sokaras D, Tian Y C, Liu Y J, Doeff M M. Thermal stress-induced charge and structure heterogeneity in emerging cathode materials[J]. Mater. Today, 2020, 35:87-98. |
| [90] | Mu L Q, Lin R L, Xu R, Han L L, Xia S H, Sokaras D, Steiner J D, Weng T C, Nordlund D, Doeff M M, Liu Y J, Zhao K J, Xin H L L, Lin F. Oxygen release induced chemomechanical breakdown of layered cathode materials[J]. Nano Lett., 2018, 18(5):3241-3249. |
| [91] | Wang C Y, Han L L, Zhang R, Cheng H, Mu L Q, Kisslinger K, Zou P C, Ren Y, Cao P H, Lin F, Xin H L. Resolving atomic-scale phase transformation and oxygen loss mechanism in ultrahigh-nickel layered cathodes for cobalt-free lithium-ion batteries[J]. Matter, 2021, 4(6):2013-2026. |
| [92] | Lv H J, Li C L, Zhao Z K, Wu B R, Mu D B. A review: Modification strategies of nickel-rich layer structure cathode (Ni ≥ 0.8) materials for lithium ion power batteries[J]. J. Energy Chem., 2021, 60:435-450. |
| [93] | Yan W W, Yang S Y, Huang Y Y, Yang Y, Yuan G H. A review on doping/coating of nickel-rich cathode materials for lithium-ion batteries[J]. J. Alloy. Compd., 2020, 819:153048. |
| [94] | Zhang S D(张思东), Liu Y(刘园), Qi M Y(祁慕尧), Cao A M(曹安民). Localized surface doping for improved stability of high energy cathode materials[J]. Acta Phys. -Chim. Sin.(物理化学学报), 2020, 37:2011007. |
| [95] | Zhao W G, Zou L F, Jia H P, Zheng J M, Wang D H, Song J H, Hong C Y, Liu R, Xu W, Yang Y, Xiao J, Wang C M, Zhang J G. Optimized al doping improves both interphase stability and bulk structural integrity of Ni-rich NMC cathode materials[J]. ACS Appl. Energ. Mater., 2020, 3(4):3369-3377. |
| [96] | Yu H F, Zhu H W, Yang Z F, Liu M M, Jiang H, Li C Z. Bulk Mg-doping and surface polypyrrole-coating enable high-rate and long-life for Ni-rich layered cathodes[J]. Chem. Eng. J., 2021, 412:128625. |
| [97] | Zhang D K, Liu Y, Wu L W, Feng L W, Jin S L, Zhang R, Jin M L. Effect of Ti ion doping on electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material[J]. Electrochim. Acta, 2019, 328:135086. |
| [98] | Choi J U, Voronina N, Sun Y K, Myung S T. Recent progress and perspective of advanced high-energy Co-less Ni-rich cathodes for Li-ion batteries: Yesterday, Today, and Tomorrow[J]. Adv. Energy Mater., 2020, 10(42):2002027. |
| [99] | Zhao Z Y, Huang B, Wang M, Yang X W, Gu Y J. Facile synjournal of fluorine doped single crystal Ni-rich cathode material for lithium-ion batteries[J]. Solid State Ionics, 2019, 342:115065. |
| [100] | Huang Z J, Wang Z X, Jing Q, Guo H J, Li X H, Yang Z H. Investigation on the effect of Na doping on structure and Li-ion kinetics of layered LiNi0.6Co0.2Mn0.2O2 cathode material[J]. Electrochim. Acta, 2016, 192:120-126. |
| [101] | Kim U H, Park G T, Son B K, Nam G W, Liu J, Kuo L Y, Kaghazchi P, Yoon C S, Sun Y K. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge[J]. Nat. Energy, 2020, 5(11):860-869. |
| [102] | Binder J O, Culver S P, Pinedo R, Weber D A, Friedrich M S, Gries K I, Volz K, Zeier W G, Janek J. Investigation of fluorine and nitrogen as anionic dopants in nickel-rich cathode materials for lithium-ion batteries[J]. ACS Appl. Mater. Inter., 2018, 10(51):44452-44462. |
| [103] | Li X L, Kang F Y, Shen W C, Bai X D. Improvement of structural stability and electrochemical activity of a cathode material LiNi0.7Co0.3O2 by chlorine doping[J]. Electrochim. Acta, 2007, 53(4):1761-1765. |
| [104] | Li J Y, Yang M X, Huang Z C, Zhao B Q, Zhang G, Li S M, Cui Y H, Dong Z H, Liu H. Nanoscale operation of Ni-rich cathode surface by polycrystalline solid electrolytes Li3.2Zr0.4Si0.6O3.6 coating[J]. Chem. Eng. J., 2021, 417:129217. |
| [105] | Li Y Y, Li X F, Hu J H, Liu W, Sari H M K, Li D J, Sun Q, Kou L, Tian Z Y, Shao L, Zhang C, Zhang J J, Sun X L. ZnO interface modified LiNi0.6Co0.2Mn0.2O2 toward boosting lithium storage[J]. Energy Environ. Mater., 2020, 3(4):522-528. |
| [106] | Cheng X P, Zheng J M, Lu J X, Li Y H, Yan P F, Zhang Y F. Realizing superior cycling stability of Ni-rich layered cathode by combination of grain boundary engineering and surface coating[J]. Nano Energy, 2019, 62:30-37. |
| [107] | Su Y F(苏岳锋), Zhang Q Y(张其雨), Chen L(陈来), Bao L(包丽颖), Lu Y(卢赟), Chen S(陈实), Wu F(吴锋). Effects of ZrO2 coating on Ni-rich LiNi0.8Co0.1Mn0.1O2 cathodes with enhanced cycle stabilities[J]. Acta Phys.Chim. Sin.(物理化学学报), 2020, 37(3):2005062. |
| [108] | Li Y, Liu X, Ren D S, Hsu H J, Xu G L, Hou J X, Wang L, Feng X N, Lu L G, Xu W Q, Ren Y, Li R H, He X M, Amine K, Ouyang M G. Toward a high-voltage fastcharging pouch cell with TiO2 cathode coating and enhanced battery safety[J]. Nano Energy, 2020, 71:104643. |
| [109] | Gan Q M, Qin N, Wang Z Y, Li Z Q, Zhu Y H, Li Y Z, Gu S, Yuan H M, Luo W, Lu L, Xu Z H, Lu Z G. Revealing mechanism of Li3PO4 coating suppressed surface oxygen release for commercial Ni-rich layered cathodes[J]. ACS Appl. Energ. Mater., 2020, 3(8):7445-7455. |
| [110] | Dai S C, Yan G J, Wang L, Luo L M, Li Y P, Yang Y T, Liu H H, Liu Y, Yuan M L. Enhanced electrochemical performance and thermal properties of Ni-rich LiNi0.8-Co0.1Mn0.1O2 cathode material via CaF2 coating[J]. J. Electroanal. Chem., 2019, 847:113197. |
| [111] | Zhang L J, Li N, Wu B R, Xu H L, Wang L, Yang X Q, Wu F. Sphere-shaped hierarchical cathode with enhanced growth of nanocrystal planes for high-rate and cycling-stable Li-ion batteries[J]. Nano Lett., 2015, 15(1):656-661. |
| [112] | Su Y F, Chen G, Chen L, Li W K, Zhang Q Y, Yang Z R, Lu Y, Bao L Y, Tan J, Chen R J, Chen S, Wu F. Exposing the {010} planes by oriented self-assembly with nanosheets to improve the electrochemical performances of Ni-rich Li[Ni0.8Co0.1Mn0.1]O2 microspheres[J]. ACS Appl. Mater. Inter., 2018, 10(7):6407-6414. |
| [113] | Hua W B, Liu W Y, Chen M Z, Indris S, Zheng Z, Guo X D, Bruns M, Wu T H, Chen Y X, Zhong B H, Chou S L, Kang Y M, Ehrenberg H. Unravelling the growth mechanism of hierarchically structured Ni1/3Co1/3Mn1/3(OH)2 and their application as precursors for high-power cathode materials[J]. Electrochim. Acta, 2017, 232:123-131. |
| [114] | Li Y C, Xiang W, Xiao Y, Wu Z G, Xu C L, Xu W, Xu Y D, Wu C, Yang Z G, Guo X D. Synergy of doping and coating induced heterogeneous structure and concentration gradient in Ni-rich cathode for enhanced electrochemical performance[J]. J. Power Sources, 2019, 423:144-151. |
| [115] | Park N Y, Ryu H H, Park G T, Noh T C, Sun Y K. Optimized Ni-rich NCMA cathode for electric vehicle batteries[J]. Adv. Energy Mater., 2021, 11(9):2003767. |
| [116] | Wu K, Wang J Y, Li Q, Yang Y Q, Deng X, Dang R B, Wu M M, Wu Z J, Xiao X L, Yu X Q. In situ synjournal of a nickel concentration gradient structure of Ni-rich LiNi0.8Co0.15Al0.05O2 with promising superior electrochemical properties at high cut-off voltage[J]. Nanoscale, 2020, 12(20):11182-11191. |
| [117] | Sun Y K, Chen Z, Noh H J, Lee D J, Jung H G, Ren Y, Wang S, Yoon C S, Myung S T, Amine K. Nanostructured high-energy cathode materials for advanced lithium batteries[J]. Nat. Mater., 2012, 11(11):942-947. |
| [118] | Yang H, Wu H H, Ge M, Li L, Yuan Y, Yao Q, Chen J, Xia L, Zheng J, Chen Z, Duan J, Kisslinger K, Zeng X C, Lee W K, Zhang Q, Lu J. Simultaneously dual modification of Ni-rich layered oxide cathode for high-energy lithium-ion batteries[J]. Adv. Funct. Mater., 2019, 29(13):1808825. |
| [1] | 左东旭, 李培超. 基于电化学-热-力耦合模型的快速充电下锂离子电池的老化特性分析[J]. 电化学(中英文), 2024, 30(9): 2402061-. |
| [2] | 方建军, 杜宇豪, 李子健, 樊文光, 任恒宇, 易浩聪, 赵庆贺, 潘锋. 高电压LiCoO2的表面结构与性能:回顾与展望[J]. 电化学(中英文), 2024, 30(6): 2314005-. |
| [3] | 陈露露, 李浩冉, 刘维祎, 王伟. 锂离子电池正极材料原位漫反射光谱电化学研究[J]. 电化学(中英文), 2024, 30(6): 2314006-. |
| [4] | 丑佳, 王雅慧, 王文鹏, 辛森, 郭玉国. 面向高性能锂-硫二次电池应用的非对称电极-电解质界面[J]. 电化学(中英文), 2023, 29(9): 2217009-. |
| [5] | 温波, 朱卓, 李福军. 锂-氧气电池:正极催化剂的最新进展与挑战[J]. 电化学(中英文), 2023, 29(2): 2215001-. |
| [6] | 赵刚, 龚正良, 李益孝, 杨勇. 氧化钨和磷钨酸对LiNi0.96Co0.02Mn0.02O2材料的表面包覆改性研究[J]. 电化学(中英文), 2023, 29(10): 2204281-. |
| [7] | 陈思, 郑淞生, 郑雷铭, 张叶涵, 王兆林. 水热法制备锂电池Si@C负极材料的工艺优化研究[J]. 电化学(中英文), 2022, 28(8): 2112221-. |
| [8] | 王京玥, 王睿, 王诗琦, 王立帆, 詹纯. 一步固相法合成锂离子电池高镍层状正极材料[J]. 电化学(中英文), 2022, 28(8): 2112131-. |
| [9] | 谯渭川, 李芳儒, 肖瑾林, 屈丽娟, 赵晓, 张梦, 庞春雷, 李子坤, 任建国, 贺雪琴. 硅氧材料的膨胀性能研究和改善[J]. 电化学(中英文), 2022, 28(5): 2108121-. |
| [10] | 胡炳文, 李超, 耿福山, 沈明. 金属离子电池中的磁共振:从核磁共振(NMR)到电子顺磁共振(EPR)[J]. 电化学(中英文), 2022, 28(2): 2108421-. |
| [11] | 郭瑞琪, 吴锋, 王欣然, 白莹, 吴川. 多电子反应材料推动高能量密度电池发展:材料与体系创新[J]. 电化学(中英文), 2022, 28(12): 2219011-. |
| [12] | 朱振威, 邱景义, 王莉, 曹高萍, 何向明, 王京, 张浩. 人工智能在锂离子电池研发中的应用[J]. 电化学(中英文), 2022, 28(12): 2219003-. |
| [13] | 李西尧, 赵长欣, 李博权, 黄佳琦, 张强. 锂硫电池复合正极研究进展[J]. 电化学(中英文), 2022, 28(12): 2219013-. |
| [14] | 侯廷政, 陈翔, 蒋璐, 唐城. 当前和下一代锂离子电池电解液的原子尺度微观认识和研究进展[J]. 电化学(中英文), 2022, 28(11): 2219007-. |
| [15] | 李丹丹, 纪翔宇, 陈明, 杨燕茹, 王晓东, 冯光. 低聚离子液体的体相与界面及其电化学储能应用[J]. 电化学(中英文), 2022, 28(11): 2219002-. |
| 阅读次数 | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
全文 4245
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
摘要 1465
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||