

碱处理对PtRu/WC电极材料结构及甲醇电催化氧化性能的影响
收稿日期: 2012-07-10
修回日期: 2012-12-24
网络出版日期: 2012-12-29
基金资助
国际科技合作计划((No. 2010DFB63680)、浙江省重大科技专项国际合作项目(No. 2008C14040)、浙江省自然科学基金重点项目(No. Z4100790)及973项目(No. 2012CB722604)资助
Influence of Pretreatment on Electrocatalytic Property for Methanol Oxidation of PtRu/WC
Received date: 2012-07-10
Revised date: 2012-12-24
Online published: 2012-12-29
郎小玲 , 施梅勤 , 江叶坤 , 马淳安 . 碱处理对PtRu/WC电极材料结构及甲醇电催化氧化性能的影响[J]. 电化学, 2013 , 19(4) : 350 -354 . DOI: 10.61558/2993-074X.2120
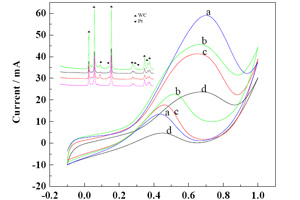
Key words: pretreatment; PtRu; tungsten carbide; catalytic performance; stability
/
| 〈 |
|
〉 |