

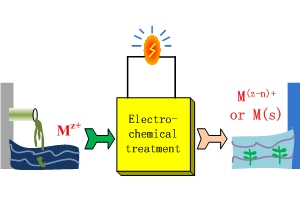
重金属自产电能的电化学处理
收稿日期: 2012-12-25
修回日期: 2013-03-15
网络出版日期: 2013-03-20
基金资助
国家自然科学基金项目(No. 21173188, No. 21073161)资助
Electrochemical Treatment of Heavy Metals with Self-Electricity Generation
Received date: 2012-12-25
Revised date: 2013-03-15
Online published: 2013-03-20
许伟 , 张慧敏 , 吴祖成 . 重金属自产电能的电化学处理[J]. 电化学, 2013 , 19(4) : 345 -349 . DOI: 10.61558/2993-074X.2965
/
| 〈 |
|
〉 |