

Pt/PMo12/PEDOT/GC电极的制备及其甲醇电氧化性能
收稿日期: 2012-03-28
修回日期: 2012-05-10
网络出版日期: 2012-05-20
基金资助
广西自然科学基金(No. 0991093)、广西教育厅科研项目(No. 201012MS024)、广西自然科学基金创新团队项目(No. 2010GXNSFF013001)和广西研究生教育创新计划资助
Preparation and Methanol Electrooxidation of Pt/PMo12/PEDOT/GC Electrodes
Received date: 2012-03-28
Revised date: 2012-05-10
Online published: 2012-05-20
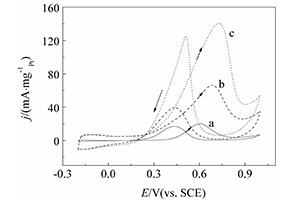
关键词: 聚3,4-乙烯二氧噻吩; 磷钼酸; 燃料电池; 阳极催化剂; 甲醇电氧化
马静华 , 王睿翔 , 谭一良 , 王珊珊 , 张艳勤 , 樊友军 . Pt/PMo12/PEDOT/GC电极的制备及其甲醇电氧化性能[J]. 电化学, 2013 , 19(2) : 164 -168 . DOI: 10.61558/2993-074X.2109

/
| 〈 |
|
〉 |