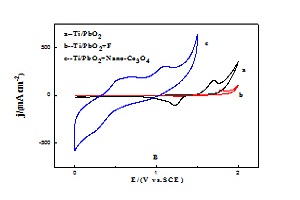
本文在SnO2-Sb2O5氧化物为中间层的钛基体上,采用电沉积法制备了无掺杂的Ti/PbO2、掺杂F的Ti/PbO2(Ti/PbO2+F)和掺杂纳米Co3O4粒子的Ti/PbO2电极(Ti/PbO2+Nano-Co3O4). 用X射线衍射(XRD)和扫描电镜(SEM)分析和观察了电极材料的组成、结构和形貌,并通过电化学方法研究了这三种电极对苯酚的电催化氧化性能. 结果表明,Ti/PbO2+F电极的析氧电位较Ti/PbO2电极的发生明显正移,但其苯酚的氧化峰和析氧峰并不能分开;而Ti/PbO2+Nano-Co3O4电极虽然其析氧电位负移,但对苯酚的氧化峰出现在析氧峰之前. 这一结果表明,体系存在着某种反应特别快的瞬态中间体,即在水分子被解离之前已与苯酚发生了反应,从而更有利于苯酚的转化和降解.
刘晓蕾
,
丹媛媛
,
陆海彦
,
林海波
,
欧阳明丽
,
袁传军
. 不同掺杂元素的钛基PbO2电极对苯酚电催化氧化性能的影响[J]. 电化学, 2013
, 19(1)
: 59
-64
.
DOI: 10.61558/2993-074X.2098
Three types of electrodes, namely, Ti/PbO2, F-doped PbO2 (Ti/PbO2-F) and nano-Co3O4-doped PbO2 (Ti/PbO2+Nano-Co3O4) electrodes, were prepared by electro-deposition method on the Ti substrate with the interlayer of SnO2-Sb2O5. X-ray diffraction (XRD) and scanning electron microscopy (SEM) were used to study the compositions, structures and film morphologies of the electrodes. Electrocatalytic oxidation characteristics to phenol on the prepared electrodes were investigated by electrochemical method. The experimental results showed that compared with the Ti/PbO2 electrode, the potentials of oxygen evolution on the Ti/PbO2+F electrode apparently shifted more positively, while those of Ti/PbO2+Nano-Co3O4 electrode shifted negatively after the oxidation of phenol took place. This provided experimental evidence for the existence of some transient intermediates which underwent particularly fast reactions, i.e., the reaction of water molecule with phenol occurred before the dissociation of water molecule, which was more beneficial to the transformation and degradation of phenol.