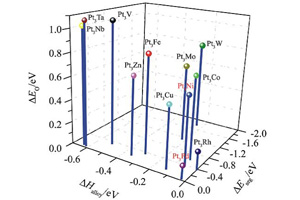
本文提出以合金形成能、Pt表面偏析能和氧原子吸附能作为依据筛选具有高活性和高稳定性的表面富Pt氧还原合金催化剂. 利用DFT计算对Pt与各种过渡金属形成的合金的热力学、表面化学和电子性质进行了系统研究,在此基础上预测Pt-V,Pt-Fe,Pt-Co,Pt-Ni,Pt-Cu,Pt-Zn,Pt-Mo,Pt-W等合金可能具有好的氧还原催化活性和稳定性. 所预期的大部分催化剂已有文献研究结果支持. 另外,Pt-Zn和Pt-Mo体系目前报道尚不多,值得进一步的细致研究.
Developing Pt-lean catalysts for oxygen reduction reaction (ORR) is the key for large-scale application of proton exchange membrane fuel cell (PEMFC). In this paper, we have proposed a multiple-descriptor strategy for screening efficient and durable ORR alloy catalysts of low Pt content. We argue that an ideal Pt-based bimetallic alloy catalyst for ORR should possess simultaneously negative alloy formation energy, negative surface segregation energy of Pt and a lower oxygen binding ability than pure Pt. By performing detailed DFT calculations on the thermodynamics, surface chemistry and electronic properties of various Pt-M alloys (M refers to non-precious transition metals in the periodic table), Pt-V,Pt-Fe,Pt-Co,Pt-Ni,Pt-Cu,Pt-Zn,Pt-Mo,Pt-W are predicted to have improved catalytic activity and durability for ORR, most of which have indeed been reported to have excellent ORR catalytic performance in the literature. It is suggested that the ORR performance of Pt-Zn and Pt-Mo systems deserve detailed theoretical and experimental investigations.
[1] Guerin S, Hayden B E, Lee C E, et al. Combinatorial electrochemical screening of fuel cell electrocatalysts[J]. Journal of Combinatorial Chemistry, 2004, 6(1): 149-158.
[2] Stamenkovic V R, Fowler B, Mun B S, et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability[J]. Science, 2007, 315(5811): 493-497.
[3] Greeley J, Mavrikakis M. Alloy catalysts designed from first-principles[J]. Nature Materials, 2004, 3(11): 810-815.
[4] Greeley J, Stephens I E L, Bondarenko A S, et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts[J]. Nature Chemistry, 2009, 1(7): 552-556.
[5] Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865-3868.
[6] Vanderbilt D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism[J]. Physical Review B, 1990, 41(11): 7892-7895.
[7] Methfessel M, Paxton A T. High-precision sampling for Brillouin-zone integration in metals[J]. Physical Review B, 1989, 40(6): 3616-3621.
[8] Baroni S, Dal Corso A, de Gironcoli S, et al. PWSCF and PHONON: Plane-wave pseudo-potential codes. http://www.pwscf.org, 2001.
[9] Mukerjee S, Srinivasan, Soriaga M P. Role of structural and electronic properties of Pt and Pt alloys on electrocatalysis of oxygen reduction[J]. Journal of the Electrochemical Society, 1995, 142(5): 1409-1422.
[10] Hammer B, N?rskov J K. Electronic factors determining the reactivity of metal surfaces[J]. Surface Science, 1995, 343(3): 211-220.
[11] Hammer B, N?rskov J K. Why gold is the noblest of all the metals[J]. Nature, 1995, 376(6537): 238-240.
[12] L?vvik O M. Surface segregation in palladium based alloys from density-functional calculations[J]. Surface Science, 2005, 583(1): 100-106.
[13] N?rskov J K, Bligaard T, Logadottir A, et al. Universality in heterogeneous catalysis[J]. Journal of Catalysis, 2002, 209(2): 275-278.
[14] Stamenkovic V R, Mun B S, Mayrhofer K J J, et al. Effect of surface composition on electronic structure, stability, and electrocatalytic properties of Pt-transition metal alloys: Pt-skin versus Pt-skeleton surfaces[J]. Journal of the American Chemical Society, 2006, 128(27): 8813-8819.
[15] Stamenkovic V R, Mun B S, Arenz M, et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces[J]. Nature Materials, 2007, 6(3): 241-247.
[16] Chen S, Ferreira P J, Sheng W C, et al. Enhanced activity for oxygen reduction reaction on “Pt3Co” nanoparticles: Direct evidence of percolated and sandwich-segregation structures[J]. Journal of the American Chemical Society, 2008, 130(42): 13818-13819.
[17] Shirlaine K, Strasser P. Electrocatalysis on bimetallic surfaces: Modifying catalytic reactivity for oxygen reduction by voltammetric surface dealloying[J]. Journal of the American Chemical Society, 2007, 129(42): 12624-12625.
[18] Dai Y, Ou L H, Liang W, et al. Efficient and superiorly durable Pt-lean electrocatalysts of Pt-W alloys for the oxygen reduction reaction[J]. Journal of Physical Chemistry C, 2011, 115(5): 2162-2168.


