

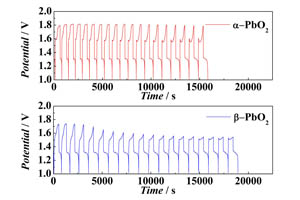
α型和β型PbO2正极的充放电性能的比较
收稿日期: 2011-11-04
修回日期: 2012-03-17
网络出版日期: 2012-03-27
基金资助
国家973项目计划(No. 2011CB935901)和山东大学自主创新基金(No. 2009JC019)资助
A Comparative Study of Charge-Discharge Behaviors of α-PbO2 and β-PbO2 Cathodes
Received date: 2011-11-04
Revised date: 2012-03-17
Online published: 2012-03-27
崔聪颖 , 马学美 , 孔德龙 , 马厚义 . α型和β型PbO2正极的充放电性能的比较[J]. 电化学, 2013 , 19(1) : 43 -52 . DOI: 10.61558/2993-074X.2938
/
| 〈 |
|
〉 |