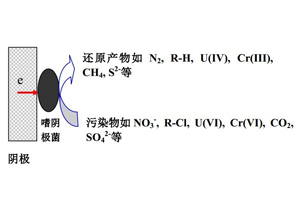
厌氧环境下一些微生物能够接受来自于电极的电子并将电子传递至环境污染物,这使得电驱动下生物还原技术在可持续性废水处理以及生物修复方面受到越来越多关注. 此体系中,阴极电子传递被认为是影响环境污染物厌氧转化可行性和效率的制约因素. 文中首先评述可能的电子传递原理,包括水解氢气介导的间接电子传递、人工合成电子穿梭体或者细菌分泌电子穿梭体介导的间接电子传递、以及电极与细菌之间的直接电子传递等途径. 相比间接电子传递,直接电子传递避免了将电子传递给没有起作用的介体及没有和电极接触的浮游微生物,因而更加节能. 另外,列举了自养反硝化、生物还原脱氯、重金属生物还原、CO2生物还原以及硫酸盐生物还原等应用实例. 最后,提出了此领域研究发展亟需解决的两个重要问题,包括阴极生物膜的培养以及电子从电极转至微生物内在机理的解析.
冯春华
,
谢道海
,
庞韵梦
,
韩涛
,
韦朝海
. 电驱动下的环境污染物厌氧生物转化—电子转移原理和应用实例[J]. 电化学, 2013
, 19(5)
: 444
-453
.
DOI: 10.61558/2993-074X.2135
The ability of some microorganisms to accept electrons from an electrode for the reduction of terminal electron acceptors in anaerobic environments has attracted growing interest on the electric field-stimulated biological reduction technology, which may open new possibility for the sustainable wastewater treatment and bioremediation in the field of environmental engineering. Here, we reviewed the extracellular electron transfer mechanism which is thought to play a key role in determining the feasibility and efficiency for the anaerobic biotransformation of environmental pollutants. Possible mechanisms that may be involved in bioelectrochemical reactors (BERs) with biocathodes include indirect electron transfer via hydrogen generated from water electrolysis or via a soluble mediator that can be artificial or secreted from bacteria, and direct transfer from the cathode to the microorganism. Direct electron transfer has many advantages over indirect electron transfer because it avoids the loss of electrons to unused mediators and planktonic cells, and thus allows significant reduction in power requirements. In addition, potential application examples of anaerobic biotransformation of environmental pollutants, known as autotrophic denitrification, microbial reductive dechlorination, heavy-metal bioreduction, CO2 bioreduction, sulfate bioreduction stimulated by an applied electric field were also reviewed. Finally, we proposed that more efforts should be made on developing new strategies for growing cathode biofilms and further disclosing biochemical mechanisms for the cathode extracellular electron transfer, in order to achieve the promising applications of this biotechnology.