

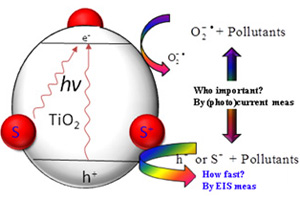
结合(光)电化学方法研究光催化降解污染物反应
收稿日期: 2012-12-25
修回日期: 2013-03-18
网络出版日期: 2013-03-18
基金资助
国家自然科学基金项目(No. 50971116)和国家基础研究计划(No. 2011CB936003)资助
An Investigation of Photocatalytic Degradation Reactions of Pollutants by Combination of (Photo)electrochemical Measurements
Received date: 2012-12-25
Revised date: 2013-03-18
Online published: 2013-03-18
冷文华 , 朱红乔 . 结合(光)电化学方法研究光催化降解污染物反应[J]. 电化学, 2013 , 19(5) : 437 -443 . DOI: 10.61558/2993-074X.2134
/
| 〈 |
|
〉 |