
电化学(中英文) ›› 2021, Vol. 27 ›› Issue (2): 185-194. doi: 10.13208/j.electrochem.201248
所属专题: “电催化和燃料电池”专题文章
秦雪苹1,*( ), 朱尚乾1, 张露露1, 孙书会2, 邵敏华1,*(
), 朱尚乾1, 张露露1, 孙书会2, 邵敏华1,*( )
)
收稿日期:2021-02-02
修回日期:2021-03-10
出版日期:2021-04-28
发布日期:2021-03-20
通讯作者:
秦雪苹,邵敏华
E-mail:xqinaa@connect.ust.hk;kemshao@ust.hk
Xue-Ping Qin1,*( ), Shang-Qian Zhu1, Lu-Lu Zhang1, Shu-Hui Sun2, Min-Hua Shao1,*(
), Shang-Qian Zhu1, Lu-Lu Zhang1, Shu-Hui Sun2, Min-Hua Shao1,*( )
)
Received:2021-02-02
Revised:2021-03-10
Published:2021-04-28
Online:2021-03-20
Contact:
Xue-Ping Qin,Min-Hua Shao
E-mail:xqinaa@connect.ust.hk;kemshao@ust.hk
摘要:
单原子催化剂(SAC)由于其低成本和在各种电催化反应中潜在的高催化活性而被认为是铂族金属的有前景的替代材料,但仍然缺乏对不同金属氮碳材料催化剂之间活性差异的原子机理的理解。在此,通过实验和理论研究相结合,研究了非贵金属氮碳材料(Me-N-C,Me = Fe和Co)作为模型催化剂,以探索在普遍的pH值下氧还原反应(ORR)和氢析出反应(HER)的催化活性以及相对应的反应机理。原子理论模拟表明,Fe-N-C具有比Co-N-C高的ORR活性,这是因为其速率决定步骤的反应势垒较低,而HER的活性趋势却相反。我们的模拟结果与实验观察结果一致。
秦雪苹, 朱尚乾, 张露露, 孙书会, 邵敏华. 酸性和碱性溶液中金属氮碳材料氧还原和氢析出反应的理论研究[J]. 电化学(中英文), 2021, 27(2): 185-194.
Xue-Ping Qin, Shang-Qian Zhu, Lu-Lu Zhang, Shu-Hui Sun, Min-Hua Shao. Theoretical Studies of Metal-N-C for Oxygen Reduction and Hydrogen Evolution Reactions in Acid and Alkaline Solutions[J]. Journal of Electrochemistry, 2021, 27(2): 185-194.
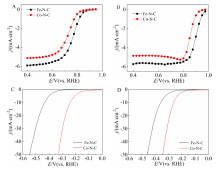
Figure 1
Steady-state ORR polarization curves of Fe-N-C and Co-N-C in O2-saturated 0.1 mol·L-1 HClO4 (A) and 0.1 mol·L-1 KOH (B) solutions. The catalyst loading was 306 μg·cm-2. The RDE rotation speed was 1600 r·min-1. Polarization curves of Fe-N-C and Co-N-C in Ar-saturated 0.5 mol·L-1 H2SO4 (C) and 1 mol·L-1 KOH (D) solutions at a scanning rate of 10 mV·s-1. The catalyst loading was 255 μg·cm-2. (color on line)
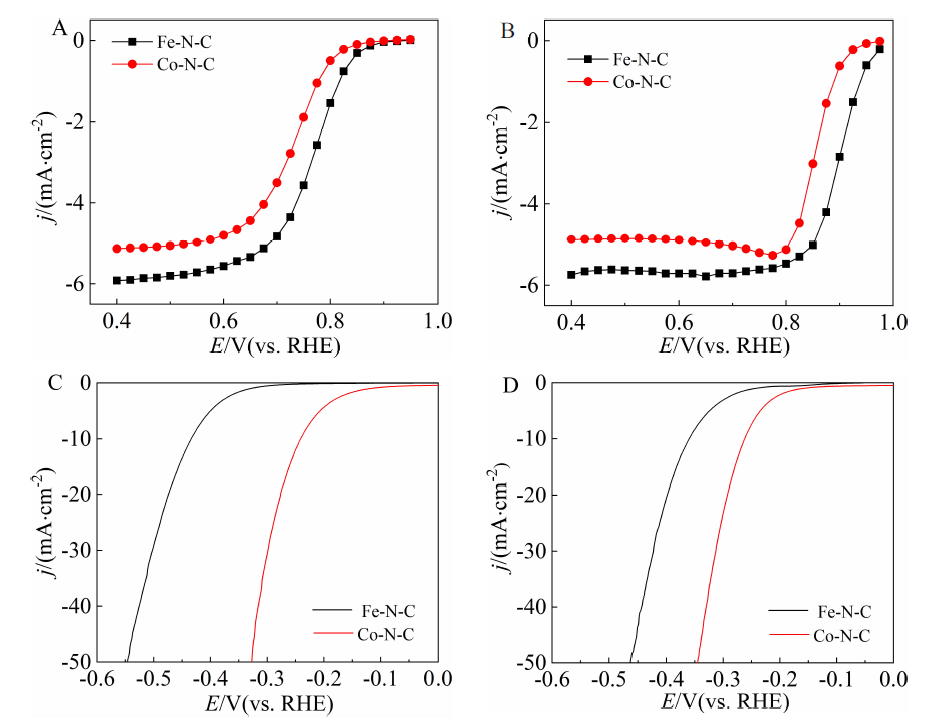

Figure 2
Top view and side view of slab model and adsorption structures of reaction intermediates during ORR on Fe-N-C: (A) slab model; (B) *OOH; (C) *O; (D) *OH. Black dashed lines in B, C and D show the weak hydrogen bonds between adsorbates and water bilayers. Reaction intermediates (*OOH, *O and *OH) are shown in ball-and-stick mode, and water molecules in the bilayer are shown in stick mode. Color code: Fe, orange; C, grey; N, dark blue; O, red; H, white. (color on line)
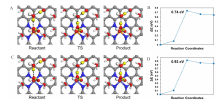
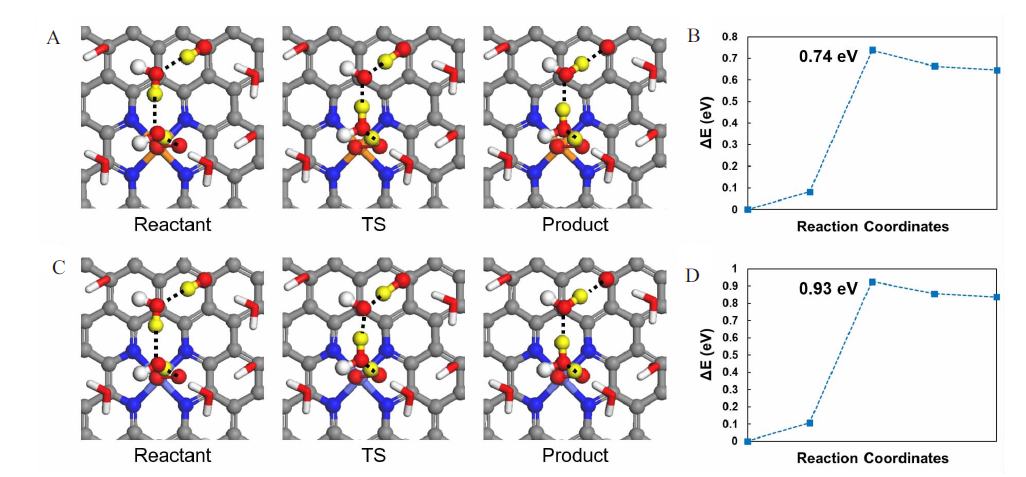
Figure 4
Optimized geometries of reactant, transition state (TS) and product in the rate-determining *OOH formation step during ORR in alkaline media and the corresponding energy curves along with TS searching on Fe-N-C (A, B) and Co-N-C (C, D). The black dashed line (B and C) indicates the proton transfer pathway, and the transferred protons are shown in the yellow balls. The involved water molecules and adsorbed intermediates are shown in ball-and-stick mode, and other water molecules are in stick mode for clarity. Color code: Fe, orange; Co, light blue; C, grey; N, dark blue; O, red; H, white. (color on line)
| [1] |
Xia B Y, Yan Y, Li N, Wu H B, Lou X W, Wang X. A metal-organic framework-derived bifunctional oxygen electrocatalyst[J]. Nat. Energy, 2016,1(1):15006.
doi: 10.1038/nenergy.2015.6 URL |
| [2] |
Ma T Y, Ran J, Dai S, Jaroniec M, Qiao S Z. Phosphorus-doped graphitic carbon nitrides grown in situ on carbon-fiber paper: Flexible and reversible oxygen electrodes[J]. Angew. Chem. Int. Ed., 2015,54(15):4646-4650.
doi: 10.1002/anie.201411125 URL |
| [3] |
Gong K P, Du F, Xia Z H, Durstock M, Dai L M. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction[J]. Science, 2009,323(5915):760-764.
doi: 10.1126/science.1168049 URL |
| [4] |
Liang Y Y, Li Y G, Wang H L, Zhou J G, Wang J, Regier T, Dai H J. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction[J]. Nat. Mater., 2011,10(10):780-786.
doi: 10.1038/nmat3087 URL |
| [5] |
Michalsky R, Zhang Y J, Peterson A A. Trends in the hydrogen evolution activity of metal carbide catalysts[J]. ACS Catal., 2014,4(5):1274-1278.
doi: 10.1021/cs500056u URL |
| [6] |
Cao B, Veith G M, Neuefeind J C, Adzic R R, Khalifah P G. Mixed close-packed cobalt molybdenum nitrides as non-noble metal electrocatalysts for the hydrogen evolution reaction[J]. J. Am. Chem. Soc., 2013,135(51):19186-19192.
doi: 10.1021/ja4081056 URL |
| [7] |
Wang H T, Lu Z Y, Xu S C, Kong D S, Cha J J, Zheng G Y, Hsu P C, Yan K, Bradshaw D, Prinz F B, Cui Y. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction[J]. Proc. Natl. Acad. Sci., 2013,110(49):19701-19706.
doi: 10.1073/pnas.1316792110 URL |
| [8] |
Wu G, More K L, Johnston C M, Zelenay P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt[J]. Science, 2011,332(6028):443-447.
doi: 10.1126/science.1200832 URL |
| [9] |
Shao M H, Chang Q W, Dodelet J P, Chenitz R. Recent advances in electrocatalysts for oxygen reduction reaction[J]. Chem. Rev., 2016,116(6):3594-3657.
doi: 10.1021/acs.chemrev.5b00462 URL |
| [10] |
Lefèvre M, Proietti E, Jaouen F, Dodelet J P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells[J]. Science, 2009,324(5923):71-74.
doi: 10.1126/science.1170051 URL |
| [11] | Zhang Y F(张焰峰), Xiao F(肖菲), Chen G Y(陈广宇), Shao M H(邵敏华). Fuel cell performance of non-precious metal based electrocatalysts[J]. J. Electrochem.(电化学) 2020,26(4):563-572. |
| [12] | Xiu L Y(修陆洋), Yu M Z(于梦舟), Yang P J(杨鹏举), Wang Z Y(王治宇), Qiu J S(邱介山). Caging porous Co-NC nanocomposites in 3D graphene as active and aggregation-resistant electrocatalyst for oxygen reduction reaction[J]. J. Electrochem.(电化学) 2018,24(6):715-725. |
| [13] |
Zhang L L, Liu W, Dou Y B, Du Z, Shao M H. The role of transition metal and nitrogen in metal-N-C composites for hydrogen evolution reaction at universal pHs[J]. J. Phys. Chem. C, 2016,120(51):29047-29053.
doi: 10.1021/acs.jpcc.6b11782 URL |
| [14] |
Shahraei A, Moradabadi A, Martinaiou I, Lauterbach S, Klemenz S, Dolique S, Kleebe H J, Kaghazchi P, Kramm U I. Elucidating the origin of hydrogen evolution reaction activity in mono- and bimetallic metal- and nitrogen-doped carbon catalysts (Me-N-C)[J]. ACS Appl. Mater. Interfaces, 2017,9(30):25184-25193.
doi: 10.1021/acsami.7b01647 URL |
| [15] |
Zhu Z J, Chen C M, Cai M Q, Cai Y, Ju H X, Hu S W, Zhang M. Porous Co-N-C ORR catalysts of high performance synthesized with ZIF-67 templates[J]. Mater. Res. Bull., 2019,114:161-169.
doi: 10.1016/j.materresbull.2019.02.029 URL |
| [16] |
Chen L Y, Liu X F, Zheng L R, Li Y C, Guo X, Wan X, Liu Q T, Shang J X, Shui J L. Insights into the role of active site density in the fuel cell performance of Co-N-C catalysts[J]. Appl. Catal. B Environ., 2019,256:117849.
doi: 10.1016/j.apcatb.2019.117849 URL |
| [17] |
Ai K L, Li Z L, Cui X Q. Scalable preparation of sized-controlled Co-N-Celectrocatalyst for efficient oxygen reduction reaction[J]. J. Power Sources, 2017,368:46-56.
doi: 10.1016/j.jpowsour.2017.09.067 URL |
| [18] | Sebastián D, Serov A, Artyushkova K, Gordon J, Atanass-ov P, Aricò A S, Baglio V. High performance and cost-effective direct methanol fuel cells: Fe-NC methanol-tolerant oxygen reduction reaction catalysts[J]. ChemSus-Chem, 2016,9(15):1986-1995. |
| [19] |
Wang Y, Pan Y, Zhu L K, Yu H H, Duan B Y, Wang R W, Zhang Z T, Qiu S L. Solvent-free assembly of Co/Fe-containing MOFs derived N-doped mesoporous carbon nanosheets for ORR and HER[J]. Carbon, 2019,146:671-679.
doi: 10.1016/j.carbon.2019.02.002 |
| [20] |
Zhang G X, Chenitz R, Lefèvre M, Sun S, Dodelet J P. Is iron involved in the lack of stability of Fe/N/C electrocatalysts used to reduce oxygen at the cathode of PEM fuel cells?[J]. Nano Energy, 2016,29:111-125.
doi: 10.1016/j.nanoen.2016.02.038 URL |
| [21] |
Zhang G X, Wei Q L, Yang X H, Tavares A C, Sun S H. RRDE experiments on noble-metal and noble-metal-free catalysts: Impact of loading on the activity and selectivity of oxygen reduction reaction in alkaline solution[J]. Appl. Catal. B Environ., 2017,206:115-126.
doi: 10.1016/j.apcatb.2017.01.001 URL |
| [22] |
Kresse G, Hafner J. Ab initio molecular dynamics for liquid metals[J]. Phys. Rev. B, 1993,47(1):558-561.
doi: 10.1103/PhysRevB.47.558 URL |
| [23] |
Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comput. Mater. Sci., 1996,6(1):15-50.
doi: 10.1016/0927-0256(96)00008-0 URL |
| [24] |
Blöchl P E. Projector augmented-wave method[J]. Phys. Rev. B, 1994,50(24):17953-17979.
doi: 10.1103/PhysRevB.50.17953 URL |
| [25] |
Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Phys. Rev. B, 1999,59(3):1758-1775.
doi: 10.1103/PhysRevB.59.1758 URL |
| [26] |
Hammer B, Hansen L B, Nørskov J K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals[J]. Phys. Rev. B, 1999,59(11):7413-7421.
doi: 10.1103/PhysRevB.59.7413 URL |
| [27] |
Monkhorst H J, Pack J D. Special points for Brillouin-zone integrations[J]. Phys. Rev. B, 1976,13(12):5188-5192.
doi: 10.1103/PhysRevB.13.5188 URL |
| [28] |
Van Den Bossche M, Skúlason E, Rose-Petruck C, Jónsson H. Assessment of constant-potential implicit solvation calculations of electrochemical energy barriers for H2 evolution on Pt[J]. J. Phys. Chem. C, 2019,123(7):4116-4124.
doi: 10.1021/acs.jpcc.8b10046 |
| [29] |
Zhang Q, Asthagiri A. Solvation effects on DFT predictions of ORR activity on metal surfaces[J]. Catal. Today, 2019,323:35-43.
doi: 10.1016/j.cattod.2018.07.036 URL |
| [30] |
Liu S Z, White M G, Liu P. Mechanism of oxygen reduction reaction on Pt(111) in alkaline solution: Importance of chemisorbed water on surface[J]. J. Phys. Chem. C, 2016,120(28):15288-15298.
doi: 10.1021/acs.jpcc.6b05126 URL |
| [31] |
Ogasawara H, Brena B, Nordlund D, Nyberg M, Pelmenschikov A, Pettersson L G M, Nilsson A. Structure and bonding of water on Pt(111)[J]. Phys. Rev. Lett., 2002,89(27):276102.
pmid: 12513221 |
| [32] |
Liu K X, Qiao Z, Hwang S, Liu Z Y, Zhang H G, Su D, Xu H, Wu G, Wang G F. Mn- and N- doped carbon as promising catalysts for oxygen reduction reaction: Theoretical prediction and experimental validation[J]. Appl. Catal. B - Environ., 2019,243:195-203.
doi: 10.1016/j.apcatb.2018.10.034 URL |
| [33] |
Mathew K, Sundararaman R, Letchworth-Weaver K, Arias T A, Hennig R G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways[J]. J. Chem. Phys., 2014,140(8):084106.
doi: 10.1063/1.4865107 URL |
| [34] |
Petrosyan S A, Rigos A A, Arias T A. Joint density-functional theory: Ab initio study of Cr2O3 surface chemistry in solution[J]. J. Phys. Chem. B, 2005,109(32):15436-15444.
pmid: 16852958 |
| [35] |
Valter M, Wickman B, Hellman A. Solvent effects for methanol electrooxidation on gold[J]. J. Phys. Chem. C, 2021,125(2):1355-1360.
doi: 10.1021/acs.jpcc.0c08923 URL |
| [36] |
Gauthier J A, Dickens C F, Heenen H H, Vijay S, Ringe S, Chan K. Unified approach to implicit and explicit solvent simulations of electrochemical reaction energetics[J]. J. Chem. Theory Comput., 2019,15(12):6895-6906.
doi: 10.1021/acs.jctc.9b00717 pmid: 31689089 |
| [37] |
Nørskov J K, Rossmeisl J, Logadottir A, Lindqvist L, Kitchin J R, Bligaard T, Jónsson H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode[J]. J. Phys. Chem. B, 2004,108(46):17886-17892.
doi: 10.1021/jp047349j URL |
| [38] | Henkelman G, Jónsson H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points[J]. J. Chem. Phys., 2000,113(22):9978-9985. |
| [39] | Henkelman G, Uberuaga B P, Jónsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths[J]. J. Chem. Phys., 2000,113(22):9901-9904. |
| [40] |
Sheppard D, Terrell R, Henkelman G. Optimization methods for finding minimum energy paths[J]. J. Chem. Phys., 2008,128(13):134106.
doi: 10.1063/1.2841941 pmid: 18397052 |
| [41] |
Chen S Q, Zhang N J, Villarrubia C W N, Huang X, Xie L, Wang X Y, Kong X D, Xu H, Wu G, Zeng J, Wang H L. Single Fe atoms anchored by short-range ordered nanographene boost oxygen reduction reaction in acidic media[J]. Nano Energy, 2019,66:104164.
doi: 10.1016/j.nanoen.2019.104164 URL |
| [42] |
Liu K X, Kattel S, Mao V, Wang G F. Electrochemical and computational study of oxygen reduction reaction on nonprecious transition metal/nitrogen doped carbon nanofibers in acid medium[J]. J. Phys. Chem. C, 2016,120(3):1586-1596.
doi: 10.1021/acs.jpcc.5b10334 URL |
| [43] |
Filhol J S, Neurock M. Elucidation of the electrochemical activation of water over Pd by first principles[J]. Angew. Chem. Int. Ed., 2006,45(3):402-406.
doi: 10.1002/(ISSN)1521-3773 URL |
| [44] |
Yeh K Y, Janik M J. Density functional theory-based electrochemical models for the oxygen reduction reaction: Comparison of modeling approaches for electric field and solvent effects[J]. J. Comput. Chem., 2011,32(16):3399-3408.
doi: 10.1002/jcc.v32.16 URL |
| [1] | 郑天龙, 欧明玉, 徐松, 毛信表, 王释一, 和庆钢. 一体式可再生燃料电池双功能氧催化剂的研究进展[J]. 电化学(中英文), 2023, 29(7): 2205301-. |
| [2] | 杨云锐, 董欢欢, 郝志强, 何祥喜, 杨卓, 李林, 侴术雷. 高性能锂硫电池用钴/碳复合材料硫宿主[J]. 电化学(中英文), 2023, 29(4): 2217003-. |
| [3] | 孟庆成, 金林薄, 马梦泽, 高学庆, 陈爱兵, 周道金, 孙晓明. 层状金属氢氧化物中铁位点辅助分散铂纳米颗粒用于高效甲醇氧化[J]. 电化学(中英文), 2023, 29(2): 2215007-. |
| [4] | 周澳, 郭伟健, 王月青, 张进涛. 焦耳热快速合成双功能电催化剂用于高效水分解[J]. 电化学(中英文), 2022, 28(9): 2214007-. |
| [5] | 张天恩, 颜雅妮, 张俊明, 瞿希铭, 黎燕荣, 姜艳霞. 调控Pt3Zn合金化程度改善酸性氧还原活性与稳定性[J]. 电化学(中英文), 2022, 28(4): 2106091-. |
| [6] | Jafar Hussain Shah, 谢起贤, 匡智崇, 格日乐, 周雯慧, 刘朵绒, Alexandre I. Rykov, 李旭宁, 罗景山, 王军虎. 原位57Fe穆斯堡尔光谱技术及其在Ni-Fe基析氧反应电催化剂中的应用[J]. 电化学(中英文), 2022, 28(3): 2108541-. |
| [7] | 王雪, 张丽, 刘长鹏, 葛君杰, 祝建兵, 邢巍. 碱性介质中非贵金属氧还原催化剂的结构调控进展[J]. 电化学(中英文), 2022, 28(2): 2108501-. |
| [8] | 魏家祺, 陈晓东, 李述周. 电化学合成纳米材料和小分子材料在电解制氢领域的应用[J]. 电化学(中英文), 2022, 28(10): 2214012-. |
| [9] | 唐佳, 张晓明, 于陕升, 王素力, 孙公权. PtxCuy/C电催化剂甲醇氧化反应性能及机理研究[J]. 电化学(中英文), 2021, 27(5): 508-517. |
| [10] | 张伟艺, 马宪印, 邹受忠, 蔡文斌. 铂和钯上丙三醇电氧化研究进展:从反应机理到催化材料[J]. 电化学(中英文), 2021, 27(3): 233-256. |
| [11] | 吴志鹏, 钟传建. 钯基氧还原和乙醇氧化反应电催化剂:关于结构和机理研究的一些近期见解[J]. 电化学(中英文), 2021, 27(2): 144-156. |
| [12] | 秦祥, 李仲秋, 潘建斌, 李剑, 王康, 夏兴华. 双极纳米电极阵列实现单个铂纳米颗粒上氢气析出反应的电致化学发光成像[J]. 电化学(中英文), 2021, 27(2): 157-167. |
| [13] | 陆杭烁, 何小波, 银凤翔, 李国儒. Ni-Fe/Ti和Ni-Fe-S/Ti的制备及其电催化水分解性能[J]. 电化学(中英文), 2020, 26(1): 136-147. |
| [14] | 张 翅, 李成飞, 李高仁. 负载于刻蚀镍泡沫上钯纳米粒子作为乙醇氧化高性能电催化剂[J]. 电化学(中英文), 2019, 25(5): 571-578. |
| [15] | 杨 帆, 邓培林, 韩优嘉, 潘 静, 夏宝玉. 电化学二氧化碳还原中的铜基催化剂[J]. 电化学(中英文), 2019, 25(4): 426-444. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||