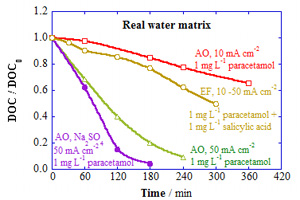
本文报告了采用掺硼金刚石阳极(BDD)电氧化和电芬顿工艺处理真实水体中注入含有少量药物残留物的单组分和多组分溶液(即1 mg·L-1对乙酰氨基酚和/或1 mg·L-1水杨酸,pH=3)的研究结果. 以恒定电流密度方式在BDD/Pt电解池中进行电氧化,而在BDD/空气扩散电解池中进行电芬顿,从而在阴极电生H2O2. 结果表明,由于乙酰氨基酚和水杨酸均与溶液中氯离子氧化所产生的活性氯物种发生反应,因此,电氧化处理真实水体中两种药物的降解要比超纯水中添加0.05 mol·L-1 Na2SO4快. 这种含氯氧化剂的反应活性甚至超过了阳极形成的有限的羟基活性基,提高电流密度大大加速了两种污染物的消除. 在真实水体自然碱性pH下得到了类似的结果. 当电氧化处理两种药物的混合物时,两种药物上面产生的氧化剂的竞争作用,导致药物的降解变慢,只有电芬顿处理真实水体时药物的降解才稍微加快,这是由于H2O2与Cl-的反应,生成了反应活性弱得多的含氯活性基,从而抑制了电生的H2O2和添加的Fe2+之间发生的芬顿反应所形成的同相羟基活性基的累积. 对于添加了药物的真实水体,在较高的电流密度下电氧化可得到较好的天然有机物成分(NOM)矿化度,且添加0.05 mol·L-1 Na2SO4效果会更好. 虽然在药物溶液的电氧化中检测出微量的氧化副产物,如对苯醌、NO3-和NH4+ 离子,但在本研究条件下无法去除真实水体中所含有的氮基化合物.
Ignasi Sirés, José Antonio Garrido, Enric Brillas
. 掺硼金刚石阳极电氧化和电芬顿工艺处理真实水体中低含量药物的研究(英文)[J]. 电化学, 2013
, 19(4)
: 300
-312
.
DOI: 10.61558/2993-074X.2963
Here, we report the performance of electro-oxidation and electro-Fenton with a boron-doped diamond (BDD) anode for the treatment of single and multicomponent solutions containing small amounts of pharmaceutical residues (i.e., 1 mg·L-1 paracetamol and/or 1 mg·L-1 salicylic acid) spiked into a real water matrix at pH 3.0. Electro-oxidation was performed in a BDD/Pt cell, whereas electro-Fenton was carried out in a BDD/air-diffusion cell to electrogenerate H2O2 at the cathode, always operating at constant current density. It was found that the decay of both pharmaceuticals by electro-oxidation was more rapid in the real water matrix than in ultrapure water with 0.05 mol·L-1 Na2SO4 because of their additional reaction with active chlorine species produced at the bulk from the oxidation of Cl ion. Such chlorinated oxidants exhibited even higher reactivity than hydroxyl radicals formed and confined at the anode. The increase in current density largely enhanced the removal of both pollutants. Similar results were found using the real water matrix at natural alkaline pH. When the mixture of both pharmaceuticals was treated by electro-oxidation, their abatement became slower owing to the competitive attack of generated oxidants over them. Only a slight acceleration of pharmaceutical decay was obtained for the real water matrix using electro-Fenton, since the accumulation of additional homogeneous hydroxyl radical formed from Fenton’s reaction between generated H2O2 and added Fe2+ was inhibited by its reaction with Cl- to form much less reactive chlorinated radicals. For the real water matrix with added pharmaceuticals, a high degree of mineralization of the natural organic matter content (NOM) was reached at high current densities by electro-oxidation, which was even improved upon addition of 0.05 mol·L-1 Na2SO4. Traces of oxidation by-products like p-benzoquinone, as well as NO3- and NH4+ ions, were detected during the electro-oxidation of paracetamol solutions, but the N-compounds contained in the real water matrix were not removed under the investigated conditions.