

CuF2/MoO3/C复合正极的电化学阻抗谱研究
收稿日期: 2012-07-16
修回日期: 2012-08-30
网络出版日期: 2012-09-10
基金资助
2012年中国矿业大学高水平论文专项基金(No. 2012LWB13)资助
An Electrochemical Impedance Spectroscopic Study of CuF2/MoO3/C Cathode Composites
Received date: 2012-07-16
Revised date: 2012-08-30
Online published: 2012-09-10
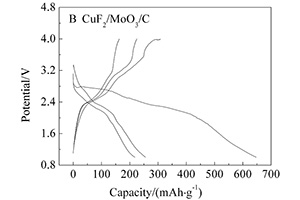
关键词: CuF2/MoO3/C复合材料; 转化反应; 电化学阻抗谱; 肖特基接触阻抗
史月丽 , 吴楠 , 沈明芳 , 董佳群 , 庄全超 , 江利 . CuF2/MoO3/C复合正极的电化学阻抗谱研究[J]. 电化学, 2013 , 19(2) : 155 -163 . DOI: 10.61558/2993-074X.2108

/
| 〈 |
|
〉 |