

超薄纳米多孔金膜的制备及其局域表面等离子体效应研究
收稿日期: 2012-03-21
修回日期: 2012-04-17
网络出版日期: 2012-04-27
基金资助
湖南省杰出青年基金(No. 09JJ1002)资助
Fabrication and Localized Surface Plasmon Resonance of Ultrathin Nanoporous Gold Films
Received date: 2012-03-21
Revised date: 2012-04-17
Online published: 2012-04-27
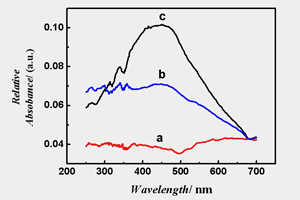
关键词: 超薄纳米多孔金膜; 电化学去合金法; 局域表面等离子体共振(LSPR)
冯时, 陈述, 杨青, 向娟 . 超薄纳米多孔金膜的制备及其局域表面等离子体效应研究[J]. 电化学, 2013 , 19(2) : 151 -154 . DOI: 10.61558/2993-074X.2107

/
| 〈 |
|
〉 |