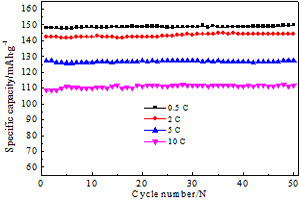
由LiH2PO4和FeC2O4.2H2O作原料、柠檬酸为碳源,用水溶-蒸发法制备了LiFePO4/C正极样品. 采用X射线衍射(XRD)、扫描电镜(SEM)和透射电镜(TEM)分析、观察样品. 用充放电曲线和电化学交流阻抗(EIS)谱图测试LiFePO4/C电极. 结果表明,700 oC焙烧的LiFePO4/C样品(碳量3.03%,by mass)结晶度高,无杂相,颗粒粒径100 nm,其表面包覆约5 nm碳层. 该电极0.5C、2C、5C和10C(1C = 170 mA.g-1)倍率放电其比容量分别为148.2 mAh.g-1、142.4 mAh.g-1、127.4 mAh.g-1和108.5 mAh.g-1,循环寿命曲线稳定.
The LiFePO4/C samples have been synthesized via an aqueous solution-evaporation route with LiH2PO4, FeC2O4.2H2O as raw materials and citric acid as a carbon source. X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) were used to analyze structure and morphology of the samples. The electrochemical performances of the LiFePO4/C cathodes were characterized by charge/discharge cures and electrochemical impedance spectroscopy (EIS). The results show that the LiFePO4/C sample, calcined at 700 °C and contained 3.03% (by mass) carbon, exhibited a highly pure crystalline phase with the primary particles sizes of 100 nm. The surfaces of those particles were covered by a carbon layer of 5 nm in thickness. At the rates of 0.5C, 2C, 5C, and 10C, where 1C corresponds to 170 mA.g-1, the discharge capacities of 148.2, 142.7, 127.4, and 108.5 mAh.g-1, were delivered, respectively, with the perfect cycling stabilities.