

基于琼脂支撑的液/液界面上AgTCNQ的电化学合成
收稿日期: 2012-07-01
修回日期: 2012-10-08
网络出版日期: 2012-10-28
基金资助
This work was supported by National Science Foundation of China (No. 20973142, No. 21061120456, and No. 21021002), National Basic Research Program of China (2011CB933700) and the National Science Foundation (CHE-1026582; MVM).
Electrochemical Synthesis of Silver-Tetracyanoquinodimethane Nanorods at Agar Supported Water/1,2-Dichloroethane Interface
Received date: 2012-07-01
Revised date: 2012-10-08
Online published: 2012-10-28
Supported by
This work was supported by National Science Foundation of China (No. 20973142, No. 21061120456, and No. 21021002), National Basic Research Program of China (2011CB933700) and the National Science Foundation (CHE-1026582; MVM).
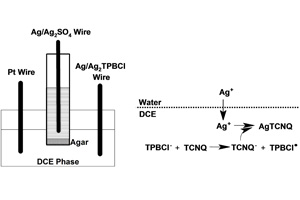
关键词: 液/液界面; 银-四氰基对苯二醌二甲烷; 电化学合成; 纳米棒
黄丽 , 汪宜娴 , Michael V. Mirkin , 任斌 , 詹东平 . 基于琼脂支撑的液/液界面上AgTCNQ的电化学合成[J]. 电化学, 2012 , 18(5) : 405 -409 . DOI: 10.61558/2993-074X.2611

/
| 〈 |
|
〉 |