

Mb/AuNPs/MWNTs/GC电极对H2O2电催化性能的研究
收稿日期: 2012-02-25
修回日期: 2012-04-10
网络出版日期: 2012-04-15
基金资助
国家863计划资助项目(No. 2008AA02Z433),国家自然科学基金资助项目(No. 20975021,No. 81171668),福建省高校产学研科技重点项目(No. 2010Y4003),福建省自然科学基金资助项目(No. 2010J06011),福建医科大学校重大项目(No. 09ZD013),福建省教育厅科技项目(No. JA10126,No. JA11110)和福建医科大学博士启动基金(No. 2011BS005)资助
Electrocatalytic Activities of Mb/AuNPs/MWNTs/GC Electrode for Hydrogen Peroxide Reduction
Received date: 2012-02-25
Revised date: 2012-04-10
Online published: 2012-04-15
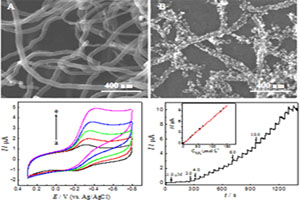
关键词: 肌红蛋白; AuNPs/MWCNTs; 直接电化学; 生物传感器
黄郑隽 , 彭花萍 , 查代君 , 刘爱林 , 陈伟 , 林新华 , 游勇基 . Mb/AuNPs/MWNTs/GC电极对H2O2电催化性能的研究[J]. 电化学, 2012 , 18(4) : 377 -382 . DOI: 10.61558/2993-074X.2934

Key words: myoglobin; AuNPs/MWNTs composite; direct electrochemistry; biosensor
/
| 〈 |
|
〉 |