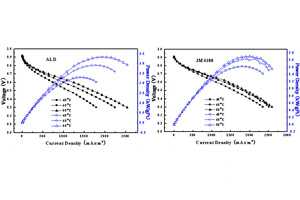
应用原子层沉积技术在碳材料复合电极基体上制备了低铂载量的高性能膜电极. 将碳载体(XC-72R)与聚四氟乙烯乳液均匀混合后涂布在碳纸上,在马弗炉中350 °C烧结,构成复合电极的基底,然后采用原子层沉积技术将铂活性组分沉积在电极基底上制得膜电极的阳极,将该阳极与经过预处理的质子交换膜及阴极压合即得膜电极. 由扫描电镜(SEM)、透射电镜(TEM)、X射线衍射(XRD)和循环伏安(CV)等分别表征该电极,单电池测试膜电极的性能. 结果表明,活性组分在阳极中高度分散,膜电极具有良好的稳定性. 膜电极的最大功率密度可达3.34 kW.(gPt)-1,是商业催化剂常规方式下制备的膜电极的1.76倍. 以本文方法制得的膜电极具有铂载量低、单位质量铂的能量密度高等特点,有望在燃料电池领域应用.
A high performance membrane electrode assembly (MEA) with low platinum loadings was successfully prepared with atomic layer deposition (ALD) technique. The anode of the MEA was prepared by depositing platinum on the carbon paper substrate, which was prepared by coating the slurry of carbon black (XC-72R) and Teflon, followed by drying and calcining at 350 °C. The MEAs consisted of the ALD anode or commercial catalyst anode, pretreated Nafion membrane (Nafion-117) and commercial cathode. Performances of MEAs were measured by single cell testing, and the anodes and MEAs were characterized by CV, SEM, TEM and XRD. The results revealed that the active component, Pt, was highly dispersed in the ALD anode and MEA with ALD anode showed excellent activity and stability. The mass activity could be high up to 3.34 kW.(gPt)-1, which is 1.76 times higher than that of the MEA with the anode prepared with commercial catalyst and conventional method. The high performance with low platinum loadings and high utilization of platinum make the ALD technique promising to be used in PEM fuel cell.