

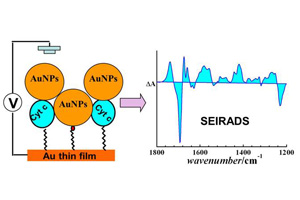
纳米金三明治结构调制细胞色素c电子传递特性的分子机理研究
收稿日期: 2011-11-29
修回日期: 2012-03-21
网络出版日期: 2012-03-25
基金资助
吉林省青年基金(No. 201101081)和中科院院长基金资助
On the Molecular Mechanism of Electron Transfer of Cytochrome c Modulated by Gold Nanoparticles in Nano-Sandwich Architecture
Received date: 2011-11-29
Revised date: 2012-03-21
Online published: 2012-03-25
蔺首睿 , 王立旭 , 姜秀娥 , 郭黎平 . 纳米金三明治结构调制细胞色素c电子传递特性的分子机理研究[J]. 电化学, 2012 , 18(3) : 263 -268 . DOI: 10.61558/2993-074X.2913
/
| 〈 |
|
〉 |