

Pt-Mo2C/GC催化甲醇氧化的现场电化学-红外光谱研究
收稿日期: 2011-12-15
修回日期: 2012-01-05
网络出版日期: 2012-01-25
基金资助
国家自然科学基金(No. 21073241, No. U1034003)资助
An In situ FTIR spectroelectrochemical study on methanol oxidation at Pt-Mo2C/GC catalyst
Received date: 2011-12-15
Revised date: 2012-01-05
Online published: 2012-01-25
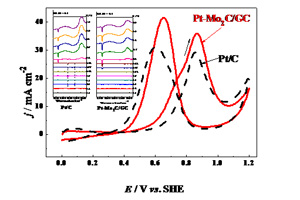
关键词: 电催化; Pt-Mo2C/GC; Pt/C; 甲醇; 现场电化学红外光谱
黄海萍 , 姚希宇 , 沈培康 . Pt-Mo2C/GC催化甲醇氧化的现场电化学-红外光谱研究[J]. 电化学, 2013 , 19(1) : 83 -88 . DOI: 10.61558/2993-074X.2939

/
| 〈 |
|
〉 |