

溶剂热法球状Ni3S4制备及其在超级电容器的应用
收稿日期: 2011-12-05
修回日期: 2011-12-12
网络出版日期: 2012-01-05
基金资助
国家自然科学基金(51171083, 51071087, 50971071)和长江学者创新团队(IRT 0927)资助
Synthesis of Spherical Ni3S4 by Solvothermal Method as Supercapacitor Electrodes
Received date: 2011-12-05
Revised date: 2011-12-12
Online published: 2012-01-05
郇庆娜 , 焦丽芳 , 王庆红 , 杜红梅 , 杨加芹 , 彭文修 , 王一菁 , 袁华堂 . 溶剂热法球状Ni3S4制备及其在超级电容器的应用[J]. 电化学, 2012 , 18(3) : 274 -277 . DOI: 10.61558/2993-074X.2915
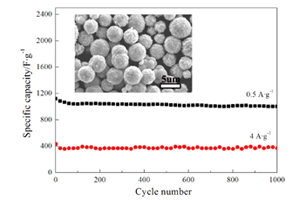
Key words: supercapacitor; nickel sulfide; solvothermal method
/
| 〈 |
|
〉 |