

阳极电沉积Ti/MnO2电极及其苯酚降解的电催化性能
收稿日期: 2011-05-05
修回日期: 2011-05-05
网络出版日期: 2011-05-06
基金资助
哈尔滨市科技创新人才研究专项资金项目(2009RFXXG204);高技术船舶专项项目
Anode Electrodeposition of Ti/MnO2 Electrode and Electrocatalytic Oxidization Properties of Phenol Degradation
Received date: 2011-05-05
Revised date: 2011-05-05
Online published: 2011-05-06
陈野* , 赵文丽 , 温青 . 阳极电沉积Ti/MnO2电极及其苯酚降解的电催化性能[J]. 电化学, 2011 , 17(2) : 199 -203 . DOI: 10.61558/2993-074X.2832
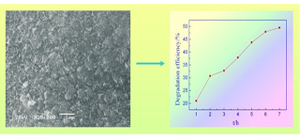
Key words: MnO2; electrodeposition; electrocatalytic oxidization; phenol
/
| 〈 |
|
〉 |