

生物质基碳负极结构调控工程实现高倍率双离子电池
Structure Regulation Engineering for Biomass-Derived Carbon Anodes Enabling High-Rate Dual-Ion Batteries
Received date: 2025-04-30
Revised date: 2025-06-08
Accepted date: 2025-06-16
Online published: 2025-06-16
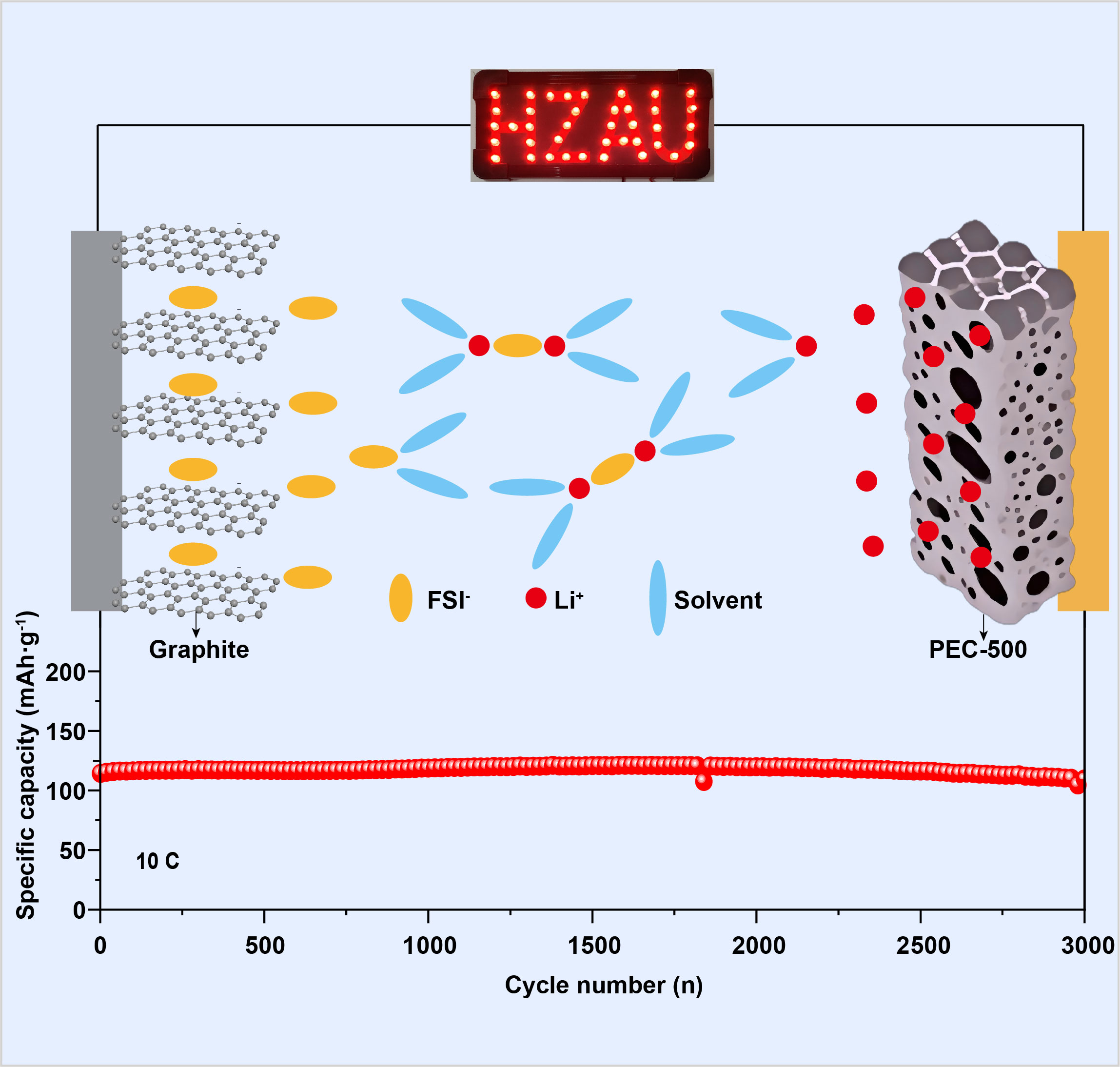
双离子电池通常使用碳基材料作为电极,具有工作电压高、潜在成本低、环境友好等优点。与二次电池传统的“摇椅式”工作机制不同,双离子电池具有独特的工作机制,在电化学反应过程中,阳离子和阴离子分别在负极和正极参与容量贡献。在高工作电压下(> 4.8 V),阴离子嵌入石墨正极具有优异的反应动力学。然而,Li+在负极侧往往扩散速率较为缓慢,导致正极和负极之间的动力学失配,严重制约了双离子电池的发展。本文通过微观结构调控策略制备了毛竹衍生碳电极材料,该策略通过选择合适的碳化温度可有效地调控其丰富的短程有序石墨微畴和无序非晶区,并具有独特的分级纳米孔结构。其纳米孔的孔径分布主要集中在0.5-5 nm,可为Li+提供合适的快速传输通道,优化后的毛竹衍生碳电极材料(碳化温度500 ℃)在300 mA·g-1电流密度下达到了436 mAh·g-1的高可逆容量和优异的倍率性能(在3 A·g-1时保持了231 mAh·g-1的高容量)。组装的双碳PEC-500||石墨全电池在10 C倍率下具有114 mAh·g-1的容量,循环3000圈后具有96%的容量保持率,同时,全电池在50 C倍率测试中仍能够保持74 mAh·g-1的容量,表现出优异的倍率性能。
周锐 , 刘瑞 , 李云诺 , 蒋思捷 , 井甜甜 , 徐艳松 , 曹菲菲 . 生物质基碳负极结构调控工程实现高倍率双离子电池[J]. 电化学, 2025 , 31(8) : 2515004 . DOI: 10.61558/2993-074X.3569
Dual-ion batteries (DIBs) usually use carbon-based materials as electrodes, showing advantages in high operating voltage, potential low cost, and environmental friendliness. Different from conventional “rocking chair” type secondary batteries, DIBs perform a unique working mechanism, which employ both cation and anion taking part in capacity contribution at an anode and a cathode, respectively, during electrochemical reactions. Graphite has been identified as a suitable cathode material for anion intercalation at high voltages (> 4.8 V) with fast reaction kinetics. However, the development of DIBs is being hindered by dynamic mismatch between a cathode and an anode due to sluggish Li+ diffusion at a high rate. Herein, we prepared phyllostachys edulis derived carbon (PEC) through microstructure regulation strategy and investigated the carbonized temperature effect, which effectively tailored the rich short-range ordered graphite microdomains and disordered amorphous regions, as well as a unique nano-pore hierarchical structure. The pore size distribution of nano-pores was concentrated in 0.5-5 nm, providing suitable channels for rapid Li+ transportation. It was found that PEC-500 (carbonized at 500 ℃) achieved a high capacity of 436 mAh·g-1 at 300 mA·g-1 and excellent rate performance (maintaining a high capacity of 231 mAh·g-1 at 3 A·g-1). The assembled dual-carbon PEC-500||graphite full battery delivered 114 mAh·g-1 at 10 C with 96% capacity retention after 3000 cycles and outstanding rate capability, providing 74 mAh·g-1 at 50 C.

| [1] | Chen S, Wu G B, Jiang H B, Wang J F, Chen T T, Han C Y, Wang W W, Yang R C, Zhao J H, Tang Z H, Gong X C, Li C F, Zhu M Y, Zhang K, Xu Y F, Wang Y, Hu Z, Chen P N, Wang B J, Zhang K, Xia Y Y, Peng H S, Gao Y. External Li supply reshapes Li deficiency and lifetime limit of batteries[J]. Nature, 2025, 638(8051): 676-683. https://doi.org/10.1038/s41586-024-08465-y. |
| [2] | Fan X L, Wang C S. High-voltage liquid electrolytes for Li batteries: progress and perspectives[J]. Chem. Soc. Rev., 2021, 50(18): 10486-10566. https://doi.org/10.1039/d1cs00450f. |
| [3] | Wan G, Pollard T P, Ma L, Schroeder M A, Chen C C, Zhu Z H, Zhang Z, Sun C J, Cai J Y, Thaman H L, Vailionis A, Li H, Kelly S, Feng Z, Franklin J, Harvey S P, Zhang Y, Du Y G, Chen Z H, Tassone C J, Steinrück H G, Xu K, Borodin O, Toney M F. Solvent-mediated oxide hydrogenation in layered cathodes[J]. Science, 2024, 385(6714): 1230-1236. https://doi.org/10.1126/science.adg4687. |
| [4] | Liu T C, Yu L, Liu J X, Dai A, Zhou T, Wang J, Huang W Y, Li L X, Li M, Li T Y, Huang X J, Xiao X H, Ge M Y, Ma L, Zhuo Z Q, Amine R, Chu Y S, Lee W K, Wen J G, Amine K. Ultrastable cathodes enabled by compositional and structural dual-gradient design[J]. Nat. Energy, 2024, 9(10): 1252-1263. https://doi.org/10.1038/s41560-024-01605-8. |
| [5] | Jin Y, Le P M L, Gao P Y, Xu Y, Xiao B W, Engelhard M H, Cao X, Vo T D, Hu J T, Zhong L R, Matthews B E, Yi R, Wang C M, Li X L, Liu J, Zhang J G. Low-solvation electrolytes for high-voltage sodium-ion batteries[J]. Nat. Energy, 2022, 7(8): 718-725. https://doi.org/10.1038/s41560-022-01055-0. |
| [6] | Liu W Y, Cui W J, Yi C J, Xia J L, Shang J B, Hu W F, Wang Z, Sang X H, Li Y Y, Liu J P. Understanding pillar chemistry in potassium-containing polyanion materials for long-lasting sodium-ion batteries[J]. Nat. Commun., 2024, 15(1): 9889. https://doi.org/10.1038/s41467-024-54317-8. |
| [7] | Park S, Wang Z L, Choudhary K, Chotard J N, Carlier D, Fauth F, Canepa P, Croguennec L, Masquelier C. Obtaining V2(PO4)3 by sodium extraction from single-phase NaxV2(PO4)3 (1<x<3) positive electrode materials[J]. Nat. Mater., 2025, 24(2): 234-242. https://doi.org/10.1038/s41563-024-02023-7. |
| [8] | Ma R F, Tao S Y, Sun X, Ren Y F, Sun C B, Ji G J, Xu J H, Wang X C, Zhang X, Wu Q W, Zhou G M. Pathway decisions for reuse and recycling of retired lithium-ion batteries considering economic and environmental functions[J]. Nat. Commun., 2024, 15(1): 7641. https://doi.org/10.1038/s41467-024-52030-0. |
| [9] | Tan S J, Tian Y F, Zhao Y, Feng X X, Zhang J, Zhang C H, Fan M, Guo J C, Yin Y X, Wang F, Xin S, Guo Y G. Noncoordinating flame-retardant functional electrolyte solvents for rechargeable lithium-ion batteries[J]. J. Am. Chem. Soc., 2022, 144(40): 18240-18245. https://doi.org/10.1021/jacs.2c08396. |
| [10] | Sabaghi D, Wang Z Y, Bhauriyal P, Lu Q Q, Morag A, Mikhailovia D, Hashemi P, Li D Q, Neumann C, Liao Z Q, Dominic A M, Nia A S, Dong R H, Zschech E, Turchanin A, Heine T, Yu M H, Feng X L. Ultrathin positively charged electrode skin for durable anion-intercalation battery chemistries[J]. Nat. Commun., 2023, 14(1): 760. https://doi.org/10.1038/s41467-023-36384-5. |
| [11] | Huang Z D, Li X L, Chen Z, Li P, Ji X L, Zhi C Y. Anion chemistry in energy storage devices[J]. Nat. Rev. Chem., 2023, 7(9): 616-631. https://doi.org/10.1038/s41570-023-00506-w. |
| [12] | Liang G J, Mo F N, Ji X L, Zhi C Y. Non-metallic charge carriers for aqueous batteries[J]. Nat. Rev. Mater., 2020, 6(2): 109-123. https://doi.org/10.1038/s41578-020-00241-4. |
| [13] | Huang Z D, Hou Y, Wang T R, Zhao Y W, Liang G J, Li X L, Guo Y, Yang Q, Chen Z, Li Q, Ma L T, Fan J, Zhi C Y. Manipulating anion intercalation enables a high-voltage aqueous dual ion battery[J]. Nat. Commun., 2021, 12(1): 3106. https://doi.org/10.1038/s41467-021-23369-5. |
| [14] | Jiang H Z, Han X Q, Du X F, Chen Z, Lu C L, Li X T, Zhang H R, Zhao J W, Han P X, Cui G L. A PF6--permselective polymer electrolyte with anion solvation regulation enabling long-cycle dual-ion battery[J]. Adv. Mater., 2022, 34(9): 2108665. https://doi.org/10.1002/adma.202108665. |
| [15] | Tong X Y, Ou X W, Wu N Z, Wang H Y, Li J, Tang Y B. High oxidation potential ≈6.0 V of concentrated electrolyte toward high-performance dual-ion battery[J]. Adv. Energy Mater., 2021, 11(25): 2100151. https://doi.org/10.1002/aenm.202100151. |
| [16] | Su Y Q, Shang J, Liu X C, Li J, Pan Q Q, Tang Y B. Constructing π-π superposition effect of tetralithium naphthalenetetracarboxylate with electron delocalization for robust dual-ion batteries[J]. Angew. Chem. Int. Ed., 2024, 63(22): e202403775. https://doi.org/10.1002/anie.202403775. |
| [17] | Xia Y, Yu F D, Nie D, Jiang Y S, Sun M Y, Que L F, Deng L, Zhao L, Zhang Q Y, Wang Z B. Unlocking fast potassium ion kinetics: High-rate and long-life potassium dual-ion battery for operation at -60 ℃[J]. Angew. Chem. Int. Ed., 2024, 63(38): e202406765. https://doi.org/10.1002/anie.202406765. |
| [18] | Wei Y K, Tang B, Liang X, Zhang F, Tang Y B. An ultrahigh-mass-loading integrated free-standing functional all-carbon positive electrode prepared using an architecture tailoring strategy for high-energy-density dual-ion batteries[J]. Adv. Mater., 2023, 35(30): 2302086. https://doi.org/10.1002/adma.202302086. |
| [19] | Liu Y J, Qiu M, Hu X, Yuan J, Liao W L, Sheng L M, Chen Y H, Wu Y M, Zhan H B, Wen Z H. Anion defects engineering of ternary Nb-based chalcogenide anodes toward high-performance sodium-based dual-ion batteries[J]. Nano-Micro Lett., 2023, 15(1): 104. https://doi.org/10.1007/s40820-023-01070-0. |
| [20] | Guo Z Y, Xu Z, Xie F, Jiang J L, Zheng K T, Alabidun S, Crespo-Ribadeneyra M, Hu Y S, Au H, Titirici M M. Investigating the superior performance of hard carbon anodes in sodium-ion compared with lithium- and potassium-ion batteries[J]. Adv. Mater., 2023, 35(42): 2304091. https://doi.org/10.1002/adma.202304091. |
| [21] | Nie L, Gao R H, Zhang M T, Zhu Y F, Wu X R, Lao Z J, Zhou G M. Integration of porous high-loading electrode and gel polymer electrolyte for high-performance quasi-solid-state battery[J]. Adv. Energy Mater., 2024, 14(4): 2302476. https://doi.org/10.1002/aenm.202302476. |
| [22] | Tang Z, Zhang R, Wang H Y, Zhou S Y, Pan Z Y, Huang Y C, Sun D, Tang Y G, Ji X B, Amine K, Shao M H. Revealing the closed pore formation of waste wood-derived hard carbon for advanced sodium-ion battery[J]. Nat. Commun., 2023, 14(1): 6024. https://doi.org/10.1038/s41467-023-39637-5. |
| [23] | Li Y Q, Vasileiadis A, Zhou Q, Lu Y X, Meng Q S, Li Y, Ombrini P, Zhao J B, Chen Z, Niu Y S, Qi X G, Xie F, van der Jagt R, Ganapathy S, Titirici M M, Li H, Chen L Q, Wagemaker M, Hu Y S. Origin of fast charging in hard carbon anodes[J]. Nat. Energy, 2024, 9(2): 134-142. https://doi.org/10.1038/s41560-023-01414-5. |
| [24] | Lian J B, Subburam G, El-Khodary S A, Zhang K, Zou B B, Wang J, Wang C, Ma J M, Wu X J. Critical role of aromatic c(sp2)-h in boosting lithium-ion storage[J]. J. Am. Chem. Soc., 2024, 146(12): 8110-8119. https://doi.org/10.1021/jacs.3c12051. |
| [25] | Zhang H H, Lin S Y, Shu C Y, Tang Z X, Wang X W, Wu Y P, Tang W. Advances and perspectives of hard carbon anode modulated by defect/hetero elemental engineering for sodium ion batteries[J]. Mater. Today, 2025, 8: 231-252. https://doi.org/10.1016/j.mattod.2025.02.014. |
| [26] | Wang J L, Huang Z J, Zhang W, Li Q H, Liang Z X, Lu J J, Lin Z Y, Wang G, Wu J X, Huang S M. Balancing graphitic nanodomains and heteroatom doping in hard carbons toward high capacity and durable potassium-ion battery anodes[J]. Adv. Funct. Mater., 2024, 34(51): 2409937. https://doi.org/10.1002/adfm.202409937. |
| [27] | Guo W S, Yu H F, Wang M, Wu M B, Chen L, Jiang H, Li C Z. Compositional gradient design of Ni-rich Co-poor cathodes enhanced cyclability and safety in high-voltage Li-ion batteries[J]. ACS Nano, 2025, 19(8): 8371-8380. https://doi.org/10.1021/acsnano.5c00974. |
| [28] | Zhang B Y, Chen L L, Zhang Z N, Li Q, Khangale P, Hildebrandt D, Liu X Y, Feng Q L, Qiao S L. Modulating the band structure of metal coordinated salen COFs and an in situ constructed charge transfer heterostructure for electrocatalysis hydrogen evolution[J]. Adv. Energy Mater., 2022, 9(22): 2105912. https://doi.org/10.1002/advs.202105912. |
| [29] | Zheng C, Jian B Q, Xu X C, Zhong J R, Yang H, Huang S M. Regulating microstructure of walnut shell-derived hard carbon for high rate and long cycling sodium-based dual-ion batteries[J]. Chem. Eng. J., 2023, 455: 140434. https://doi.org/10.1016/j.cej.2022.140434. |
| [30] | Chen C, Huang Y, Zhu Y D, Zhang Z, Guang Z X, Meng Z Y, Liu P B. Nonignorable influence of oxygen in hard carbon for sodium ion storage[J]. ACS Sustain. Chem. Eng., 2020, 8(3): 1497-1506. https://doi.org/10.1021/acssuschemeng.9b05948. |
| [31] | Han C J, Wang H Y, Wang Z L, Ou X W, Tang Y B. Solvation structure modulation of high-voltage electrolyte for high-performance K-based dual-graphite battery[J]. Adv. Mater., 2023, 35(24): 2300917. https://doi.org/10.1002/adma.202300917. |
| [32] | Xiang L, Ou X W, Wang X Y, Zhou Z M, Li X, Tang Y B. Highly concentrated electrolyte towards enhanced energy density and cycling life of dual-ion battery[J]. Angew. Chem. Int. Ed., 2020, 59(41): 17924-17930. https://doi.org/10.1002/anie.202006595. |
| [33] | Du L Y, Zhang Y M, Xiao Y Y, Yuan D, Yao M, Zhang Y. A defect-rich carbon induced built-in interfacial electric field accelerating ion-conduction towards superior-stable solid-state batteries[J]. Energy Environ. Sci., 2025, 18(6): 2949-2961. https://doi.org/10.1039/d4ee05966b. |
/
| 〈 |
|
〉 |