

锌-碘电池的挑战与机遇:从电极材料设计到储能机理
Invited Contributions from Award Winners 22nd National Electrochemical Congress in 2024
收稿日期: 2025-05-14
修回日期: 2025-06-02
录用日期: 2025-06-16
网络出版日期: 2025-06-16
Bridging Materials and Energy Storage Mechanisms in Zn-I2 Batteries
Received date: 2025-05-14
Revised date: 2025-06-02
Accepted date: 2025-06-16
Online published: 2025-06-16
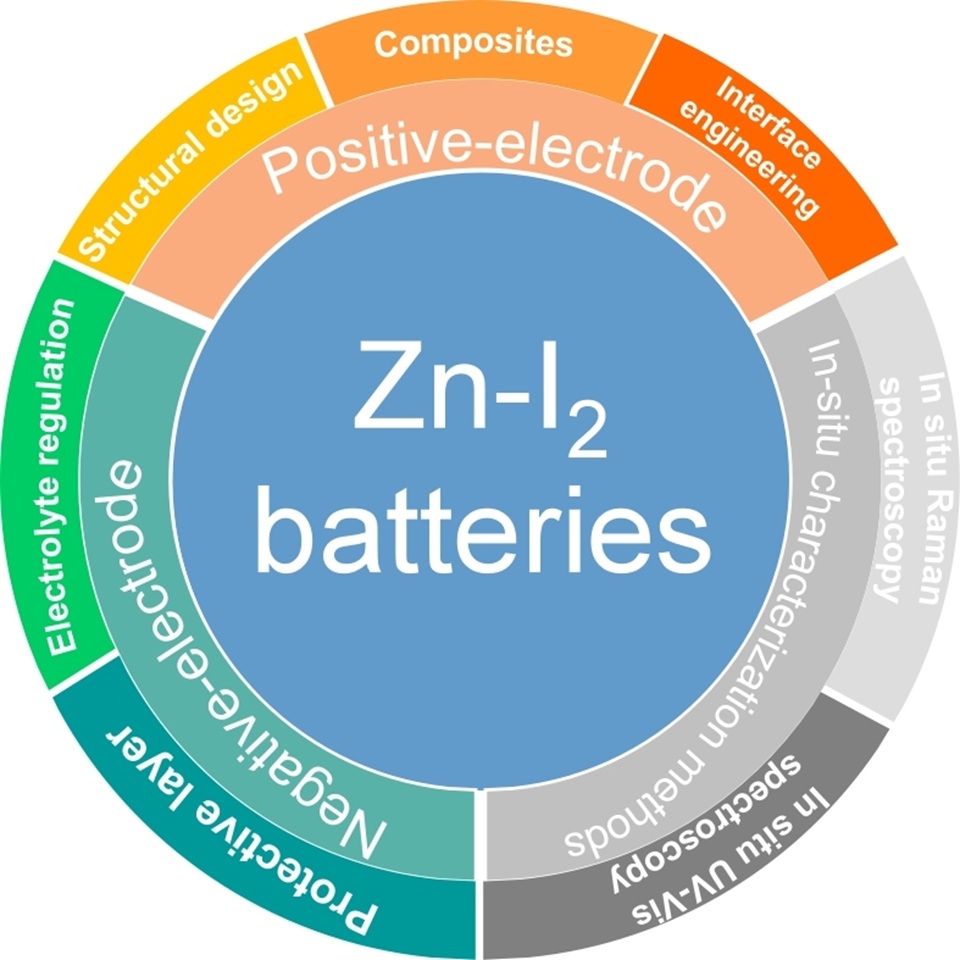
锌-碘(Zn-I2)电池因其高能量密度和环境友好性而备受关注,成为一种可持续的储能解决方案。本综述系统总结了Zn-I2电池在三个关键领域的最新研究进展:正极材料工程、锌负极稳定性调控和电解液设计。在正极方面,通过将碘锚定于导电基体上,可有效抑制多碘阴离子的穿梭效应,并加速I-/I2的氧化还原反应动力学。借助先进的原位表征技术,实现了对多碘中间体(I3-/I5-)的实时监测,深入揭示了电解质与电极间的相互作用,并为功能性添加剂的设计提供了理论依据,有效抑制了穿梭效应。在锌负极方面,近年来的创新手段如界面保护层的构建、三维导电骨架的引入及针对性的电解液添加剂的开发,在抑制锌枝晶生长和副反应方面取得了显著成效,从而提升了循环稳定性和库仑效率。然而,要在实际应用条件下实现长期可逆性和结构完整性仍面临诸多挑战。未来的研究应聚焦于协同电解液体系的构建和集成化电极结构的设计,以在化学稳定性、离子传输能力与机械强度之间实现协同优化,推动下一代Zn-I2电池技术的应用。特别是,开发具有协同增效作用的多功能电解质添加剂,以及构建兼顾力学强度与离子传输动力学的复合电极结构,将在提升Zn-I2电池性能和深化储能机理理解方面发挥关键作用。
刘榕麒 , 商文硕 , 张进涛 . 锌-碘电池的挑战与机遇:从电极材料设计到储能机理[J]. 电化学, 2025 , 31(9) : 2515005 . DOI: 10.61558/2993-074X.3567
Zinc-iodine (Zn-I2) batteries have emerged as a compelling candidate for large-scale energy storage, driven by the growing demand for safe, cost-effective, and sustainable alternatives to conventional systems. Benefiting from the inherent advantages of aqueous electrolytes and zinc metal anodes, including high ionic conductivity, low flammability, natural abundance, and high volumetric capacity, Zn-I2 batteries offer significant potential for grid-level deployment. This review provides a comprehensive overview of recent progress in three critical domains: positive-electrode engineering, zinc anode stabilization, and in situ characterization methods. On the cathode side, anchoring iodine to conductive matrices effectively mitigates polyiodide shuttling and enhances the kinetics of I-/I2 conversion. Advanced in situ characterization has enabled real-time monitoring of polyiodide intermediates (I3-/I5-), offering new insights into electrolyte-electrode interactions and guiding the development of functional additives to suppress shuttle effects. For the zinc anode, innovations such as protective interfacial layers, three-dimensional host frameworks, and targeted electrolyte additives have shown efficacy in suppressing dendrite growth and side reactions, thus improving cycling stability and coulombic efficiency. Despite these advances, challenges remain in achieving long-term reversibility and structural integrity under practical conditions. Future directions include the design of synergistic electrolyte systems, and integrated electrode architectures that simultaneously optimize chemical stability, ion transport and mechanical durability for next-generation Zn-I2 battery technologies.

Key words: Zinc-iodine battery; Interface chemistry; Dendrite growth; Shuttle effect
| [1] | Wu M J, Zhang G X, Yang H M, Liu X H, Dubois M, Gauthier M A, Sun S H. Aqueous Zn-based rechargeable batteries: Recent progress and future perspectives[J]. InfoMat, 2022, 4(5): e12265. https://doi.org/10.1002/inf2.12265 |
| [2] | Castro V, Georgiou M, Jackson T, Hodgkinson I R, Jackson L, Lockwood S. Digital data demand and renewable energy limits: Forecasting the impacts on global electricity supply and sustainability[J]. Energy Policy, 2024, 195: 114404. https://doi.org/10.1016/j.enpol.2024.114404 |
| [3] | Sarr C T, Camara M B, Dakyo B. Supercapacitors aging assessment in wind/tidal intermittent energies application with variable temperature[J]. J. Energy Storage, 2022, 46: 103790. https://doi.org/10.1016/j.est.2021.103790 |
| [4] | Yamaki A, Fujii S, Kanematsu Y, Kikuchi Y. Comparative life cycle assessment of energy storage systems for connecting large-scale wind energy to the grid[J]. J. Chem. Eng. Jpn., 2024, 57(1): 2406316. https://doi.org/10.1080/00219592.2024.2406316 |
| [5] | Mainar A R, Colmenares L C, Blázquez J A, Urdampilleta I. A brief overview of secondary zinc anode development: The key of improving zinc‐based energy storage systems[J]. Int. J. Energy Res., 2018, 42(3): 903-918. https://doi.org/10.1002/er.3822 |
| [6] | Li H F, Ma L T, Han C P, Wang Z F, Liu Z X, Tang Z J, Zhi C Y. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives[J]. Nano Energy, 2019, 62: 550-587. https://doi.org/10.1016/j.nanoen.2019.05.059 |
| [7] | Zhang Y, Wei C L, Wu M-X, Wang Y, Jiang H, Zhou G H, Tang X, Liu X M. A high-performance COF-based aqueous zinc-bromine battery[J]. Chem. Eng. J., 2023, 451: 138915. https://doi.org/10.1016/j.cej.2022.138915 |
| [8] | Yamamoto T, Hishinuma M, Yamamoto A. Zn|ZnI2|Iodine Secondary battery using Iodine-Nylon-6 adduct as positive electrode, and its charge-discharge performance[J]. Inorg. Chim. Acta, 1984, 86(2): 47-49. https://doi.org/10.1016/S0020-1693(00)82320-8 |
| [9] | Li Z H, Tan J, Wang Y, Gao C Y, Wang Y G, Ye M X, Shen J F. Building better aqueous Zn-organic batteries[J]. Energ. Environ. Sci., 2023, 16(6): 2398-2431. https://doi.org/10.1039/d3ee00211j |
| [10] | Sparkes C, Lacey N K. A study of mercuric oxide and zinc-air battery life in hearing aids[J]. J. Laryngol. Otol., 1997, 111(9): 814-819. https://doi.org/10.1017/s002221510013871x |
| [11] | Xu C J, Li B H, Du H D, Kang F Y. Energetic zinc ion chemistry: the rechargeable zinc ion battery[J]. Angew Chem. Int. Ed., 2012, 51(4): 933-935. https://doi.org/10.1002/anie.201106307 |
| [12] | Liu Y, He G, Jiang H, Parkin I P, Shearing P R, Brett D J L. Cathode design for aqueous rechargeable multivalent ion batteries: challenges and opportunities[J]. Adv. Funct Mater., 2021, 31(13): 2010445. https://doi.org/10.1002/adfm.202010445 |
| [13] | Yamamoto T, Shoji T. Rechargeable Zn|ZnSO4|MnO2-type cells[J]. Inorg. Chim. Acta, 1986, 117(2): 27-28. https://doi.org/10.1016/S0020-1693(00)82175-1 |
| [14] | Jia H, Wang Z Q, Tawiah B, Wang Y D, Chan C Y, Fei B, Pan F. Recent advances in zinc anodes for high-performance aqueous Zn-ion batteries[J]. Nano Energy, 2020, 70: 104523. https://doi.org/10.1016/j.nanoen.2020.104523 |
| [15] | Yang Q, Liang G J, Guo Y, Liu Z X, Yan B X, Wang D H, Huang Z D, Li X L, Fan J, Zhi C Y. Do zinc dendrites exist in neutral zinc batteries: A developed electrohealing strategy to in situ rescue in‐service batteries[J]. Adv. Mater., 2019, 31(43): 1903778. https://doi.org/10.1002/adma.201903778 |
| [16] | Chen H, Li X, Fang K Q, Wang H Y, Ning J Q, Hu Y. Aqueous zinc-iodine batteries: From electrochemistry to energy storage mechanism[J]. Adv. Energy Mater., 2023, 13(41): 2302187. https://doi.org/10.1002/aenm.202302187 |
| [17] | Innocenti A, Bresser D, Garche J, Passerini S. A critical discussion of the current availability of lithium and zinc for use in batteries[J]. Nat. Commun., 2024, 15(1): 4068. https://doi.org/10.1038/s41467-024-48368-0 |
| [18] | Zhou T, Gao G. V2O5-based cathodes for aqueous zinc ion batteries: Mechanisms, preparations, modifications, and electrochemistry[J]. Nano Energy, 2024, 127: 109691. https://doi.org/10.1016/j.nanoen.2024.109691 |
| [19] | Li Y X, Zhao J X, Hu Q, Hao T W, Cao H, Huang X M, Liu Y, Zhang Y Y, Lin D M, Tang Y X, Cai Y Q. Prussian blue analogs cathodes for aqueous zinc ion batteries[J]. Mater. Today Energy, 2022, 29: 101095. https://doi.org/10.1016/j.mtener.2022.101095 |
| [20] | Li D J, Guo Y X, Zhang C X, Chen X H, Zhang W S, Mei S L, Yao C-J. Unveiling organic electrode materials in aqueous zinc-ion batteries: From structural design to electrochemical performance[J]. Nano-Micro Lett., 2024, 16(1): 194. https://doi.org/10.1007/s40820-024-01404-6 |
| [21] | Zeng X M, Meng X J, Jiang W, Liu J, Ling M, Yan L J, Liang C D. Anchoring polyiodide to conductive polymers as cathode for high-performance aqueous zinc-iodine batteries[J]. ACS Sustainable Chem. Eng., 2020, 8(38): 14280-14285. https://doi.org/10.1021/acssuschemeng.0c05283 |
| [22] | Ma J Z, Liu M M, He Y L, Zhang J T. Iodine redox chemistry in rechargeable batteries[J]. Angew Chem. Int. Ed., 2021, 60(23): 12636-12647. https://doi.org/10.1002/anie.202009871 |
| [23] | Svensson P H, Kloo L. Synthesis, structure, and bonding in polyiodide and metal iodide-iodine systems[J]. Chem. Rev., 2003, 103(5): 1649-1684. https://doi.org/10.1021/cr0204101 |
| [24] | Dang H X, Sellathurai A J, Barz D P. An Ion Exchange Membrane-Free, Ultrastable zinc-iodine battery enabled by functionalized graphene electrodes[J]. Energy Storage Mater., 2023, 55: 680-690. https://doi.org/10.1016/j.ensm.2022.12.033 |
| [25] | Zhang S J, Hao J N, Li H, Zhang P F, Yin Z W, Li Y Y, Zhang B K, Lin Z, Qiao S Z. Polyiodide confinement by starch enables shuttle‐free Zn-iodine batteries[J]. Adv. Mater., 2022, 34(23): 2201716. https://doi.org/10.1002/adma.202201716 |
| [26] | Yang Q, Li Q, Liu Z X, Wang D H, Guo Y, Li X L, Tang Y C, Li H F, Dong B B, Zhi C Y. Dendrites in Zn‐based batteries[J]. Adv. Mater., 2020, 32(48): 2001854. https://doi.org/10.1002/adma.202001854 |
| [27] | Wang J W, Yang Y, Zhang Y X, Li Y M, Sun R, Wang Z C, Wang H. Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries[J]. Energy Storage Mater., 2021, 35: 19-46. https://doi.org/10.1016/j.ensm.2020.10.027 |
| [28] | Zhang L Q, Guo H L, Zong W, Huang Y P, Huang J J, He G J, Liu T X, Hofkens J, Lai F L. Metal-iodine batteries:: Achievements, challenges, and future[J]. Energ. Environ. Sci., 2023, 16(11): 4872-4925. https://doi.org/10.1039/d3ee01677c |
| [29] | Zhang W W, Wang M L, Ma J K, Zhang H, Fu L, Song B, Lu S T, Lu K. Bidirectional atomic iron catalysis of sulfur redox conversion in high‐energy flexible Zn-S battery[J]. Adv. Funct. Mater., 2023, 33(11): 2210899. https://doi.org/10.1002/adfm.202210899 |
| [30] | Shi W, Lee W S V, Xue J. Recent development of Mn‐based oxides as zinc-ion battery cathode[J]. ChemSusChem, 2021, 14(7): 1634-1658. https://doi.org/10.1002/cssc.202002493 |
| [31] | Zhang Y R, Chen A B, Sun J. Promise and challenge of vanadium-based cathodes for aqueous zinc-ion batteries[J]. J. Energy Chem., 2021, 54: 655-667. https://doi.org/10.1016/j.jechem.2020.06.013 |
| [32] | Wan F, Zhang L L, Wang X Y, Bi S S, Niu Z Q, Chen J. An aqueous rechargeable zinc-organic battery with hybrid mechanism[J]. Adv. Funct. Mater., 2018, 28(45): 1804975. https://doi.org/10.1002/adfm.201804975 |
| [33] | Sun J Q, Ma H L, Wang D W. Heavily heteroatoms doped carbons with tunable microstructure as the iodine hosts for rechargeable zinc-iodine aqueous batteries[J]. J. Alloy. Compd., 2023, 947: 169696. https://doi.org/10.1016/j.jallcom.2023.169696 |
| [34] | Tian H J, Gao T, Li X G, Wang X W, Luo C, Fan X L, Yang C Y, Suo L M, Ma Z H, Han W I. High power rechargeable magnesium/iodine battery chemistry[J]. Nat. Commun., 2017, 8(1): 14083. https://doi.org/10.1038/ncomms14083 |
| [35] | Wang Y L, Sun Q L, Zhao Q Q, Cao J S, Ye S H. Rechargeable lithium/iodine battery with superior high-rate capability by using iodine-carbon composite as cathode[J]. Energ. Environ. Sci., 2011, 4(10): 3947-3950. https://doi.org/10.1039/C1EE01875B |
| [36] | Lu K, Hu Z Y, Ma J Z, Ma H Y, Dai L M, Zhang J T. A rechargeable iodine-carbon battery that exploits ion intercalation and iodine redox chemistry[J]. Nat. Commun., 2017, 8(1): 527. https://doi.org/10.1038/s41467-017-00649-7 |
| [37] | Kim S, Kim S-K, Sun P, Oh N, Braun P V. Reduced graphene oxide/LiI composite lithium ion battery cathodes[J]. Nano Lett., 2017, 17(11): 6893-6899. https://doi.org/10.1021/acs.nanolett.7b03290 |
| [38] | Cai F S, Duan Y Q, Yuan Z H. Iodine/β-cyclodextrin composite cathode for rechargeable lithium-iodine batteries[J]. J Mater. Sci-Mater. El., 2018, 29: 11540-11545. https://doi.org/10.1007/s10854-018-9249-z |
| [39] | Zhang Q, Wu Z Z, Liu F, Liu S, Liu J, Wang Y L, Yan T Y. Encapsulating a high content of iodine into an active graphene substrate as a cathode material for high-rate lithium-iodine batteries[J]. J. Mater. Chem. A, 2017, 5(29): 15235-15242. https://doi.org/10.1039/C7TA04246A |
| [40] | Yu H X, Wang Z, Zheng R T, Yan L, Zhang L Y, Shu J. Toward sustainable metal-iodine batteries: Materials, electrochemistry and design strategies[J]. Angew. Chem. Int. Ed., 2023, 62(46): e202308397. https://doi.org/10.1002/anie.202308397 |
| [41] | Pan H L, Li B, Mei D H, Nie Z M, Shao Y Y, Li G S, Li X S, Han K S, Mueller K T, Sprenkle V, Liu J. Controlling solid-liquid conversion reactions for a highly reversible aqueous zinc-iodine battery[J]. ACS Energy Lett., 2017, 2(12): 2674-2680. https://doi.org/10.1021/acsenergylett.7b00851 |
| [42] | Wang H F, Zhang G M, Ke L L, Liu B D, Zhang S, Deng C. Understanding the effects of 3D porous architectures on promoting lithium or sodium intercalation in iodine/C cathodes synthesized via a biochemistry-enabled strategy[J]. Nanoscale, 2017, 9(27): 9365-9375. https://doi.org/10.1039/C7NR02311A |
| [43] | Lu K, Zhang H, Ye F L, Luo W, Ma H Y, Huang Y H. Rechargeable potassium-ion batteries enabled by potassium-iodine conversion chemistry[J]. Energy Storage Mater., 2019, 16: 1-5. https://doi.org/10.1016/j.ensm.2018.04.018 |
| [44] | Gong D C, Wang B, Zhu J Y, Podila R, Rao A M, Yu X Z, Xu Z, Lu B G. An iodine quantum dots based rechargeable sodium-iodine battery[J]. Adv. Energy Mater., 2017, 7(3): 1601885. https://doi.org/10.1002/aenm.201601885 |
| [45] | Marcus Y. Ionic radii in aqueous solutions[J]. Chem. Rev., 1988, 88(8): 1475-1498. https://doi.org/10.1021/cr00090a003 |
| [46] | Li Z G, Wu X H, Yu X Y, Zhou S Y, Qiao Y, Zhou H, Sun S G. Long-life aqueous Zn-I2 battery enabled by a low-cost multifunctional zeolite membrane separator[J]. Nano Lett., 2022, 22(6): 2538-2546. https://doi.org/10.1021/acs.nanolett.2c00460 |
| [47] | Li Y X, Liu L J, Li H X, Cheng F Y, Chen J. Rechargeable aqueous zinc-iodine batteries: pore confining mechanism and flexible device application[J]. Chem. Commun., 2018, 54(50): 6792-6795. https://doi.org/10.1039/c8cc02616e |
| [48] | Lee J H, Srimuk P, Fleischmann S, Ridder A, Zeiger M, Presser V. Nanoconfinement of redox reactions enables rapid zinc iodide energy storage with high efficiency[J]. J. Mater. Chem. A, 2017, 5(24): 12520-7. https://doi.org/10.1039/c7ta03589f |
| [49] | Zhang S J, Hao J N, Li H, Zhang P F, Yin Z W, Li Y Y, Zhang B K, Lin Z, Qiao S Z. Polyiodide confinement by starch enables shuttle-free Zn-iodine batteries[J]. Adv. Mater., 2022, 34(23): 2201716. https://doi.org/10.1002/adma.202201716 |
| [50] | Wang H, Shao Y, Mei S L, Lu Y, Zhang M, Sun J-K, Matyjaszewski K, Antonietti M, Yuan J Y. Polymer-derived heteroatom-doped porous carbon materials[J]. Chem. Rev., 2020, 120(17): 9363-9419. https://doi.org/10.1021/acs.chemrev.0c00080 |
| [51] | Liu W F, Liu P G, Lyu Y H, Wen J, Hao R, Zheng J Y, Liu K Y, Li Y J, Wang S. Advanced Zn-I2 battery with excellent cycling stability and good rate performance by a multifunctional iodine host[J]. ACS Appl. Mater. Interfaces, 2022, 14(7): 8955-8962. https://doi.org/10.1021/acsami.1c21026 |
| [52] | Liu T T, Wang H J, Lei C J, Mao Y, Wang H Q, He X, Liang X. Recognition of the catalytic activities of graphitic N for zinc-iodine batteries[J]. Energy Storage Mater, 2022, 53: 544-551. https://doi.org/10.1016/j.ensm.2022.09.028 |
| [53] | Chai S B, Yao J J, Wang Y L, Zhu J H, Jiang J. Mediating iodine cathodes with robust directional halogen bond interactions for highly stable rechargeable Zn-I2 batteries[J]. Chem. Eng. J., 2022, 439: 135676. https://doi.org/10.1016/j.cej.2022.135676 |
| [54] | Han L, Huang H L, Li J F, Yang Z L, Zhang X L, Zhang D F, Liu X J, Xu M, Pan L K. Novel Zinc-iodine hybrid supercapacitors with a redox iodide ion electrolyte and B, N dual-doped carbon electrode exhibit boosted energy density[J]. J. Mater. Chem. A, 2019, 7(42): 24400-244007. https://doi.org/10.1039/c9ta07196b |
| [55] | Chen Q W, Chen S, Ma J Z, Ding S Y, Zhang J T. Synergic anchoring of Fe2N nanoclusters on porous carbon to enhance reversible conversion of iodine for high-temperature zinc-iodine battery[J]. Nano Energy, 2023, 117: 108897. https://doi.org/10.1016/j.nanoen.2023.108897 |
| [56] | Yu D L, Kumar A, Nguyen T A, Nazir M T, Yasin G. High-voltage and ultrastable aqueous zinc-iodine battery enabled by N-doped carbon materials: Revealing the contributions of nitrogen configurations[J]. ACS Sustain, Chem. Eng., 2020, 8(36): 13769-13776. https://doi.org/10.1021/acssuschemeng.0c04571 |
| [57] | Ma L T, Ying Y R, Chen S M, Huang Z D, Li X L, Huang H T, Zhi C Y. Electrocatalytic iodine reduction reaction enabled by aqueous zinc‐iodine battery with improved power and energy densities[J]. Angew. Chem. Int. Ed., 2020, 60(7): 3791-3798. https://doi.org/10.1002/anie.202014447 |
| [58] | Li X L, Li N, Huang Z D, Chen Z, Liang G J, Yang Q, Li M, Zhao Y W, Ma L T, Dong B B, Huang Q, Fan J, Zhi C Y. Enhanced redox kinetics and duration of aqueous I2/I- conversion chemistry by MXene confinement[J]. Adv. Mater., 2021, 33(8): 2006897. https://doi.org/10.1002/adma.202006897 |
| [59] | Gao W Z, Cheng S T, Zhang Y X, Xie E Q, Fu J C. Efficient charge storage in zinc-iodine batteries based on pre‐embedded iodine‐ions with reduced electrochemical reaction barrier and suppression of polyiodide self‐shuttle effect[J]. Adv. Funct. Mater., 2023, 33(17): 2211979. https://doi.org/10.1002/adfm.202211979 |
| [60] | Yang G Z, Liang Z H, Li Q, Li Y, Tian F, Wang C X. Epitaxial core-shell MnFe prussian blue cathode for highly stable aqueous zinc batteries[J]. ACS Energy Lett., 2023, 8(10): 4085-4095. https://doi.org/10.1021/acsenergylett.3c01603 |
| [61] | Yan W B, Liu Y, Qiu J Z, Tan F P, Liang J H, Cai X Z, Dai C L, Zhao J Q, Lin Z F. A tripartite synergistic optimization strategy for zinc-iodine batteries[J]. Nat. Commun., 2024, 15(1): 9702. https://doi.org/10.1038/s41467-024-53800-6 |
| [62] | Chen S, Ma J Z, Chen Q W, Shang W S, Liu J S, Zhang J T. Exploring interfacial electrocatalysis for iodine redox conversion in zinc-iodine battery[J]. Sci. Bull., 2025, 70(4): 546-555. https://doi.org/10.1016/j.scib.2024.11.042 |
| [63] | Wu B K, Mu Y B, He J F, Li M, Li Z, Chu Y Q, Li Y J, Zeng L. In situ characterizations for aqueous rechargeable zinc batteries[J]. Carbon Neutralization, 2023, 2(3): 310-338. https://doi.org/10.1002/cnl2.56 |
| [64] | Zhang P F, Li J H, Zhang S J, Li D C, Zeng S Y, Xu S L, Yao Q X, Liu L Y, Ding L, Li H X, Hu Y Y, Li J T, Zhou Y. Toward shuttle‐free Zn-I2 battery: Anchoring and catalyzing iodine conversion by high-density P‐doping sites in carbon host[J]. Adv. Funct. Mater., 2023, 34(3): 2306359. https://doi.org/10.1002/adfm.202306359 |
| [65] | Jin X T, Song L, Dai C L, Xiao Y K, Han Y Y, Li X Y, Wang Y, Zhang J T, Zhao Y, Zhang Z P, Chen N, Jiang L, Qu L T. A flexible aqueous zinc-iodine microbattery with unprecedented energy density[J]. Adv. Mater., 2022, 34(15): 2109450. https://doi.org/10.1002/adma.202109450 |
| [66] | Guo C F, Cao Y N, Gao Y, Zhi C W, Wang Y X, Luo Y H, Yang X J, Luo X P. Cobalt Single-atom electrocatalysts enhanced by hydrogen‐bonded organic frameworks for long-lasting zinc‐iodine batteries[J]. Adv. Funct. Mater., 2024, 34(18): 2314189. https://doi.org/10.1002/adfm.202314189 |
| [67] | Lyu Y Q, Yuwono J A, Wang P T, Wang Y Y, Yang F H, Liu S L, Zhang S L, Wang B F, Davey K, Mao J F, Guo Z P. Organic pH buffer for dendrite-free and shuttle-free Zn-I2 batteries[J]. Angew. Chem. Int. Ed., 2023, 62(21): e202303011. https://doi.org/10.1002/anie.202303011 |
| [68] | Feng Z Y, Feng Y, Fan F F, Deng D Z, Dong H, Liu S D, Kang L, Jun S C, Wang L, Zhu J, Dai L, He Z X. Functionalization design of zinc anode for advanced aqueous zinc‐ion batteries[J]. SusMat, 2024, 4(2): e184. https://doi.org/10.1002/sus2.184 |
| [69] | Jia X X, Liu C F, Neale Z G, Yang J H, Cao G Z. Active materials for aqueous zinc ion batteries: synthesis, crystal structure, morphology, and electrochemistry[J]. Chem. Rev., 2020, 120(15): 7795-7866. https://doi.org/10.1021/acs.chemrev.9b00628 |
| [70] | Zhou M, Guo S, Fang G Z, Sun H M, Cao X X, Zhou J, Pan A Q, Liang S Q. Suppressing by-product via stratified adsorption effect to assist highly reversible zinc anode in aqueous electrolyte[J]. J. Energy Chem., 2021, 55: 549-556. https://doi.org/10.1016/j.jechem.2020.07.021 |
| [71] | Wang A R, Zhou W J, Huang A X, Chen M F, Chen J Z, Tian Q H, Xu J L. Modifying the Zn anode with carbon black coating and nanofibrillated cellulose binder: A strategy to realize dendrite-free Zn-MnO2 batteries[J]. J. Colloid Interface Sci., 2020, 577: 256-264. https://doi.org/10.1016/j.jcis.2020.05.102 |
| [72] | Li Z, Wu L, Dong S, Xu T, Li S, An Y, Jiang J, Zhang X. Pencil drawing stable interface for reversible and durable aqueous zinc-ion batteries[J]. Adv. Funct. Mater., 2021, 31(4): 2006495. https://doi.org/10.1002/adfm.202006495 |
| [73] | Yang Q, Guo Y, Yan B X, Wang C D, Liu Z X, Huang Z D, Wang Y K, Li Y R, Li H F, Song L, Fan J, Zhi C Y. Hydrogen‐substituted graphdiyne ion tunnels directing concentration redistribution for commercial‐grade dendrite‐free zinc anodes[J]. Adv. Mater., 2020, 32(25): 2001755. https://doi.org/10.1002/adma.202001755 |
| [74] | Dong L B, Yang W, Yang W, Tian H, Huang Y F, Wang X L, Xu C J, Wang C Y, Kang F Y, Wang G X. Flexible and conductive scaffold-stabilized zinc metal anodes for ultralong-life zinc-ion batteries and zinc-ion hybrid capacitors[J]. Chem. Eng. J., 2020, 384: 123355. https://doi.org/10.1016/j.cej.2019.123355 |
| [75] | Xia A L, Pu X M, Tao Y Y, Liu H M, Wang Y G. Graphene oxide spontaneous reduction and self-assembly on the zinc metal surface enabling a dendrite-free anode for long-life zinc rechargeable aqueous batteries[J]. Appl. Surf. Sci., 2019, 481: 852-859. https://doi.org/10.1016/j.apsusc.2019.03.197 |
| [76] | Li W, Wang K L, Zhou M, Zhan H C, Cheng S J, Jiang K. Advanced low-cost, high-voltage, long-life aqueous hybrid sodium/zinc batteries enabled by a dendrite-free zinc anode and concentrated electrolyte[J]. ACS Appl. Mater. Interfaces, 2018, 10(26): 22059-22066. https://doi.org/10.1021/acsami.8b04085 |
| [77] | Zheng J X, Zhao Q, Tang T, Jiefu Yin, Calvin D. Quilty, Genesis D. Renderos, Xiaotun Liu, Yue Deng, Lei Wang, David C. Bock, Cherno Jaye, Duhan Zhang, Esther S. Takeuchi3, Kenneth J. Takeuchi, Amy C. Marschilok, Archer L A. Reversible epitaxial electrodeposition of metals in battery anodes[J]. Science, 2019, 366(6465): 645-648. https://doi.org/10.1126/science.aax6873 |
| [78] | Zhou J H, Xie M, Wu F, Mei Y, Hao Y T, Huang R L, Wei G G, Liu A N, Li L, Chen R J. Ultrathin surface coating of nitrogen‐doped graphene enables stable zinc anodes for aqueous zinc‐ion batteries[J]. Adv. Mater., 2021, 33(33): 2101649. https://doi.org/10.1002/adma.202101649 |
| [79] | Cui M W, Xiao Y, Kang L, Du W, Gao Y F, Sun X Q, Zhou Y L, Li X M, Li H F, Jiang F Y, Zhi C Y. Quasi-isolated Au particles as heterogeneous seeds to guide uniform Zn deposition for aqueous zinc-ion batteries[J]. ACS Appl. Energy Mater., 2019, 2(9): 6490-6496. https://doi.org/10.1021/acsaem.9b01063 |
| [80] | Zheng J X, Liu X, Zheng Y G, Gandi A N, Kuai X X, Wang Z C, Zhu Y P, Zhuang Z C, Liang H F. AgxZny protective coatings with selective Zn2+/H+ binding enable reversible Zn anodes[J]. Nano Lett., 2023, 23(13): 6156-6163. https://doi.org/10.1021/acs.nanolett.3c01706 |
| [81] | Li B Y, Yang K, Ma J B, Shi P R, Chen L K, Chen C M, Hong X, Cheng X, Tang M C, He Y B, Kang F Y. Multicomponent copper-zinc alloy layer enabling ultra-stable zinc metal anode of aqueous Zn-ion battery[J]. Angew. Chem. Int. Ed., 2022, 61(47): e202212587. https://doi.org/10.1002/anie.202212587 |
| [82] | Fayette M, Chang H J, Li X L, Reed D. High-performance InZn alloy anodes toward practical aqueous zinc batteries[J]. ACS Energy Lett., 2022, 7(6): 1888-1895. https://doi.org/10.1021/acsenergylett.2c00843 |
| [83] | Lu M, Xiao B H, Lu Y X, Xiao K, Liu Z Q. Structural design and interface modification with selective H+ binding of 3D zinc anode for aqueous zinc‐ion batteries[J]. Adv. Energy Mater., 2025: 2500785. https://doi.org/10.1002/aenm.202500785 |
| [84] | Yi R J, Shi X D, Tang Y, Yang Y Q, Zhou P, Lu B G, Zhou J. Carboxymethyl chitosan-modified zinc anode for high-performance zinc-iodine battery with narrow operating voltage[J]. Small Struct., 2023, 4(9): 2300020. https://doi.org/10.1002/sstr.202300020 |
| [85] | Wang K, Le J B, Zhang S J, Ren W F, Yuan J M, Su T T, Chi B Y, Shao C Y, Sun R C. A renewable biomass-based lignin film as an effective protective layer to stabilize zinc metal anodes for high-performance zinc-iodine batteries[J]. J. Mater. Chem. A, 2022, 10(9): 4845-4857. https://doi.org/10.1039/D1TA10170F |
| [86] | Shang W S, Li Q, Jiang F Y, Huang B K, Song J S, Yun S, Liu X, Kimura H, Liu J J, Kang L T. Boosting Zn||I2 battery’s performance by coating a zeolite-based cation-exchange protecting layer[J]. Nano-Micro Lett., 2022, 14(1): 82. https://doi.org/10.1007/s40820-022-00825-5 |
| [87] | Shu C, An Y L, Liu Y Q, Xu Y L, Ren D Y, Zhang X Y, Sun J C, Ma Z J, Huang Y F, Kang F Y, Jiang F Y, Liu W B. Construction of corrosion-resistant and dendrite-free zinc anode by coating nano-ceriumoxide for highly stable zinc battery[J]. Chem. Eng. J., 2025, 509: 161096. https://doi.org/10.1016/j.cej.2025.161096 |
| [88] | Liu M Q, Yang L Y, Liu H, Amine A, Zhao Q H, Song Y L, Yang J L, Wang K, Pan F. Artificial solid-electrolyte interface facilitating dendrite-free zinc metal anodes via nanowetting effect[J]. ACS Appl. Mater. Interfaces, 2019, 11(35): 32046-32051. https://doi.org/10.1021/acsami.9b11243 |
| [89] | Guo Z, Fan L, Zhao C, Chen A, Liu N, Zhang Y, Zhang N. A dynamic and self-adapting interface coating for stable Zn-metal anodes[J]. Adv. Mater., 2022, 34(2): 2105133. https://doi.org/10.1002/adma.202105133 |
| [90] | Wang S J, Yang Z, Chen B T, Zhou H, Wan S F, Hu L Z, Qiu M, Qie L, Yu Y. A highly reversible, dendrite-free zinc metal anodes enabled by a dual-layered interface[J]. Energy Storage Mater., 2022, 47: 491-499. https://doi.org/10.1016/j.ensm.2022.02.024 |
| [91] | Liu J S, Chen S, Shang W S, Ma J Z, Zhang J T. In Situ Formation of 3D ZIF-8/MXene composite coating for high-performance zinc-iodine batteries[J]. Adv. Funct. Mater., 2025, 35(19): 2422081. https://doi.org/10.1002/adfm.202422081 |
| [92] | Zhao R R, Yang Y, Liu G X, Zhu R J, Huang J B, Chen Z Y, Gao Z H, Chen X, Qie L. Redirected Zn electrodeposition by an anti‐corrosion elastic constraint for highly reversible Zn anodes[J]. Adv. Funct. Mater., 2020, 31(2): 2001867. https://doi.org/10.1002/adfm.202001867 |
| [93] | Zhang Q, Luan J Y, Huang X B, Wang Q, Sun D, Tang Y G, Ji X B, Wang H Y. Revealing the role of crystal orientation of protective layers for stable zinc anode[J]. Nat. Commun., 2020, 11(1): 3961. https://doi.org/10.1038/s41467-020-17752-x |
| [94] | Wang X, Meng J P, Lin X G, Yang Y D, Zhou S, Wang Y P, Pan A Q. Stable zinc metal anodes with textured crystal faces and functional zinc compound coatings[J]. Adv. Funct. Mater., 2021, 31(48): 2106114. https://doi.org/10.1002/adfm.202106114 |
| [95] | Chen A, Zhao C Y, Guo Z K, Lu X Y, Liu N N, Zhang Y, Fan L H, Zhang N Q. Fast-growing multifunctional ZnMoO4 protection layer enable dendrite-free and hydrogen-suppressed Zn anode[J]. Energy Storage Mater, 2022, 44: 353-359. https://doi.org/10.1016/j.ensm.2021.10.016 |
| [96] | Zhao R, Yang Y, Liu G, Zhu R, Huang J, Chen Z, Gao Z, Chen X, Qie L. Redirected Zn electrodeposition by an anti-corrosion elastic constraint for highly reversible Zn anodes[J]. Adv. Funct. Mater., 2021, 31(2): 2001867. https://doi.org/10.1002/adfm.202001867 |
| [97] | Qiu N, Chen H, Yang Z M, Sun S, Wang Y. Low-cost birnessite as a promising cathode for high-performance aqueous rechargeable batteries[J]. Electrochim. Acta, 2018, 272: 154-160. https://doi.org/10.1016/j.electacta.2018.04.012 |
| [98] | Pan H L, Shao Y Y, Yan P F, Cheng Y W, Han K S, Nie Z M, Wang C M, Yang J H, Li X L, Bhattacharya P, Mueller K T, Liu J. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions[J]. Nat. Energy, 2016, 1(5): 16039. https://doi.org/10.1038/nenergy.2016.39 |
| [99] | Zeng X H, Liu J, Mao J F, Hao J N, Wang Z J, Zhou S, Ling C D, Guo Z P. Toward a reversible Mn4+/Mn2+ redox reaction and dendrite-free Zn anode in near-neutral aqueous Zn/MnO2 batteries via salt anion chemistry[J]. Adv. Energy Mater., 2020, 10(32): 1904163. https://doi.org/10.1002/aenm.201904163 |
| [100] | Xu X N, Song M, Li M, Xu Y, Sun L M, Shi L L, Su Y Q, Lai C, Wang C. A novel bifunctional zinc gluconate electrolyte for a stable Zn anode[J]. Chem. Eng. J., 2023, 454: 140364. https://doi.org/10.1016/j.cej.2022.140364 |
| [101] | Yang G S, Huang J L, Wan X H, Liu B B, Zhu Y C, Wang J W, Fontaine O, Luo S Q, Hiralal P, Guo Y Z, Zhou H. An aqueous zinc‐ion battery working at -50 °C enabled by low‐concentration perchlorate‐based chaotropic salt electrolyte[J]. EcoMat, 2022, 4(2): e12165. https://doi.org/10.1002/eom2.12165 |
| [102] | Wang F, Borodin O, Gao T, Fan X L, Sun W, Han F D, Faraone A, Dura J A, Xu K, Wang C. Highly reversible zinc metal anode for aqueous batteries[J]. Nat. Mater., 2018, 17(6): 543-549. https://doi.org/10.1038/s41563-018-0063-z |
| [103] | Olbasa B W, Huang C J, Fenta F W, Jiang S K, Chala S A, Tao H C, Nikodimos Y, Wang C C, Sheu H S, Yang Y W, Ma T L, Wu S H, Su W N, Dai H, Hwang B J. Highly reversible Zn metal anode stabilized by dense and anion-derived passivation layer obtained from concentrated hybrid aqueous electrolyte[J]. Adv. Funct. Mater., 2022, 32(7): 2103959. https://doi.org/10.1002/adfm.202103959 |
| [104] | Liu S C, He J F, Liu D S, Ye M H, Zhang Y F, Qin Y L, Li C C. Suppressing vanadium dissolution by modulating aqueous electrolyte structure for ultralong lifespan zinc ion batteries at low current density[J]. Energy Storage Mater., 2022, 49: 93-101. https://doi.org/10.1016/j.ensm.2022.03.038 |
| [105] | Cao J, Zhang D D, Zhang X Y, Zeng Z Y, Qin J Q, Huang Y H. Strategies of regulating Zn2+ solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries[J]. Energ. Environ, Sci., 2022, 15(2): 499-528. https://doi.org/10.1039/d1ee03377h |
| [106] | Wan F, Zhang Y, Zhang L L, Liu D B, Wang C D, Song L, Niu Z Q, Chen J. Reversible oxygen redox chemistry in aqueous zinc‐ion batteries[J]. Angew. Chem. Int. Ed., 2019, 58(21): 7062-7067. https://doi.org/10.1002/anie.201902679 |
| [107] | Song X Y, He H B, Shiraz M H A, Zhu H Z, Khosrozadeh A, Liu J. Enhanced reversibility and rlectrochemical window of Zn-ion batteries with an acetonitrile/water-in-salt electrolyte[J]. Chem. Commun., 2021, 57(10): 1246-1249. https://doi.org/10.1039/D0CC06076C |
| [108] | Xu W N, Zhao K N, Huo W C, Wang Y Z, Yao G, Gu X, Cheng H W, Mai L Q, Hu C G, Wang X D. Diethyl ether as self-healing electrolyte additive enabled long-life rechargeable aqueous zinc ion batteries[J]. Nano Energy, 2019, 62: 275-281. https://doi.org/10.1016/j.nanoen.2019.05.042 |
| [109] | Liu L Y, Lu H Y, Han C, Chen X F, Liu S C, Zhang J K, Chen X G, Wang X Y, Wang R, Xu J T, Liu H K, Dou S X, Li W J. Salt anion amphiphilicity-activated electrolyte cosolvent selection strategy toward durable Zn metal anode[J]. ACS Nano, 2023, 17(22): 23065-23078. https://doi.org/10.1021/acsnano.3c08716 |
| [110] | Yang T Y, Su T T, Wang H L, Li K, Ren W F, Sun R C. “Tennis racket” hydrogel electrolytes to synchronously regulate cathode and anode of zinc-iodine batteries[J]. J. Energy Chem., 2025, 102: 454-462. https://doi.org/10.1016/j.jechem.2024.11.004 |
| [111] | Cao S C, Zhang A W, Fang H Y, Feng B J, Liu Y S, Yi P S, He S, Ren Z H, Ma L L, Lu W Y, Ye M X, Shen J F. Skin-like quasi-solid-state electrolytes for spontaneous zinc-ion dehydration toward ultra-stable zinc-iodine batteries[J]. Energ. Environ, Sci., 2025, 18(7): 3395-3406. https://doi.org/10.1039/D4EE05527F |
| [112] | Shang J H, Wang Y X, Chen S, Zhang J. Solvation structure regulation of zinc ions with nitrogen-heterocyclic additives for advanced batteries[J]. Nanoscale, 2025, 17(4): 2121-2129. https://doi.org/10.1039/D4NR03776F |
| [113] | Chen S, Ji D L, Chen Q W, Ma J Z, Hou S Q, Zhang J T. Coordination modulation of hydrated zinc ions to enhance redox reversibility of zinc batteries[J]. Nat. Commun., 2023, 14(1): 3526. https://doi.org/10.1038/s41467-023-39237-3 |
| [114] | Wang K X, He Y T, Yuan R D, Chen Z Y, Gou Q Z, Zhang S D, Mei H P, Zheng Y J, Wang J, Li M. A bioimmune mechanism-inspired targeted elimination mechanism on the anode interface for zinc-iodine batteries[J]. Chem. Sci., 2025, 16(17): 7227-7238. https://doi.org/10.1039/D5SC00040H |
| [115] | Zhang L Q, Zhang M J, Guo H L, Tian Z H, Ge L F, He G J, Huang J J, Wang J T, Liu T X, Parkin I P, Lai F L. A universal polyiodide regulation using quaternization engineering toward high value-added and ultra-stable zinc-iodine batteries[J]. Adv. Sci., 2022, 9(13): 2105598. https://doi.org/10.1002/advs.202105598 |
| [116] | Song C L, Gong Z S, Bai C, Cai F S, Yuan Z H, Liu X Z. High performance Zn-I2 battery with acetonitrile electrolyte working at low temperature[J]. Nano Res., 2022, 15(4): 3170-3177. https://doi.org/10.1007/s12274-021-3884-z |
| [117] | Guo C F, Han B, Sun W W, Cao Y N, Zhang Y F, Wang Y. Hydrogen-bonded organic framework for high-performance lithium/sodium-iodine organic batteries[J]. Angew. Chem. Int. Ed., 2022, 61(48): e202213276. https://doi.org/10.1002/anie.202213276 |
| [118] | Bai C, Jin H J, Gong Z S, Liu X Z, Yuan Z H. A high-power aqueous rechargeable Fe-I2 battery[J]. Energy Storage Mater., 2020, 28: 247-254. https://doi.org/10.1016/j.ensm.2020.03.010 |
| [119] | Huang I, Zhang Y M, Arafa H M, Li S P, Vazquez-Guardado A, Ouyang W, Liu F, Madhvapathy S, Song J W, Tzavelis A, Trueb J, Choi Y, Jeang W J, Forsberg V, Higbee-Dempsey E, Ghoreishi-Haack N, Stepien I, Bailey K, Han S L, Zhang Z J, Good C, Huang Y, Bandodkar A J, Rogers J A. High performance dual-electrolyte magnesium-iodine batteries that can harmlessly resorb in the environment or in the body[J]. Energ. Environ, Sci., 2022, 15(10): 4095-4108. https://doi.org/10.1039/d2ee01966c |
| [120] | Yu D, Kumar A, Tuan Anh N, Nazir M T, Yasin G. High-voltage and ultrastable aqueous zinc-iodine battery enabled by N-doped carbon materials: Revealing the contributions of nitrogen configurations[J]. ACS Sustain. Chem. Eng, 2020, 8(36): 13769-13776. https://doi.org/10.1021/acssuschemeng.0c04571 |
| [121] | Xu J W, Ma W Q, Ge L H, Ren M M, Cai X X, Liu W L, Yao J S, Zhang C B, Zhao H. Confining iodine into a biomass-derived hierarchically porous carbon as cathode material for high performance zinc-iodine battery[J]. J. Alloys Compd., 2022, 912: 165151. https://doi.org/10.1016/j.jallcom.2022.165151 |
| [122] | Liu W, Liu P, Lyu Y, Wen J, Hao R, Zheng J, Liu K, Li Y-J, Wang S. Advanced Zn-I2 battery with excellent cycling stability and good rate performance by a multifunctional iodine host[J]. ACS Appl. Mater. Interfaces, 2022, 14(7): 8955-8962. https://doi.org/10.1021/acsami.1c21026 |
| [123] | Shang W S, Zhu J H, Liu Y, Kang L T, Liu S Y, Huang B K, Song J S, Li X M, Jiang F Y, Du W, Gao Y F, Luo H J. Establishing high-performance quasi-solid Zn/I2 Batteries with alginate-based hydrogel electrolytes[J]. ACS Appl. Mater. Interfaces, 2021, 13(21): 24756-24764. https://doi.org/10.1021/acsami.1c03804 |
/
| 〈 |
|
〉 |