

富氧空位的超亲水多孔CoOOH纳米结构用于大电流密度尿素电氧化
收稿日期: 2025-03-23
修回日期: 2025-04-30
录用日期: 2025-05-20
网络出版日期: 2025-05-19
Superhydrophilic Porous CoOOH Nano-Architecture with Abundant Oxygen Vacancies for Enhanced Urea Electrooxidation at Ampere-Level Current Densities
Received date: 2025-03-23
Revised date: 2025-04-30
Accepted date: 2025-05-20
Online published: 2025-05-19
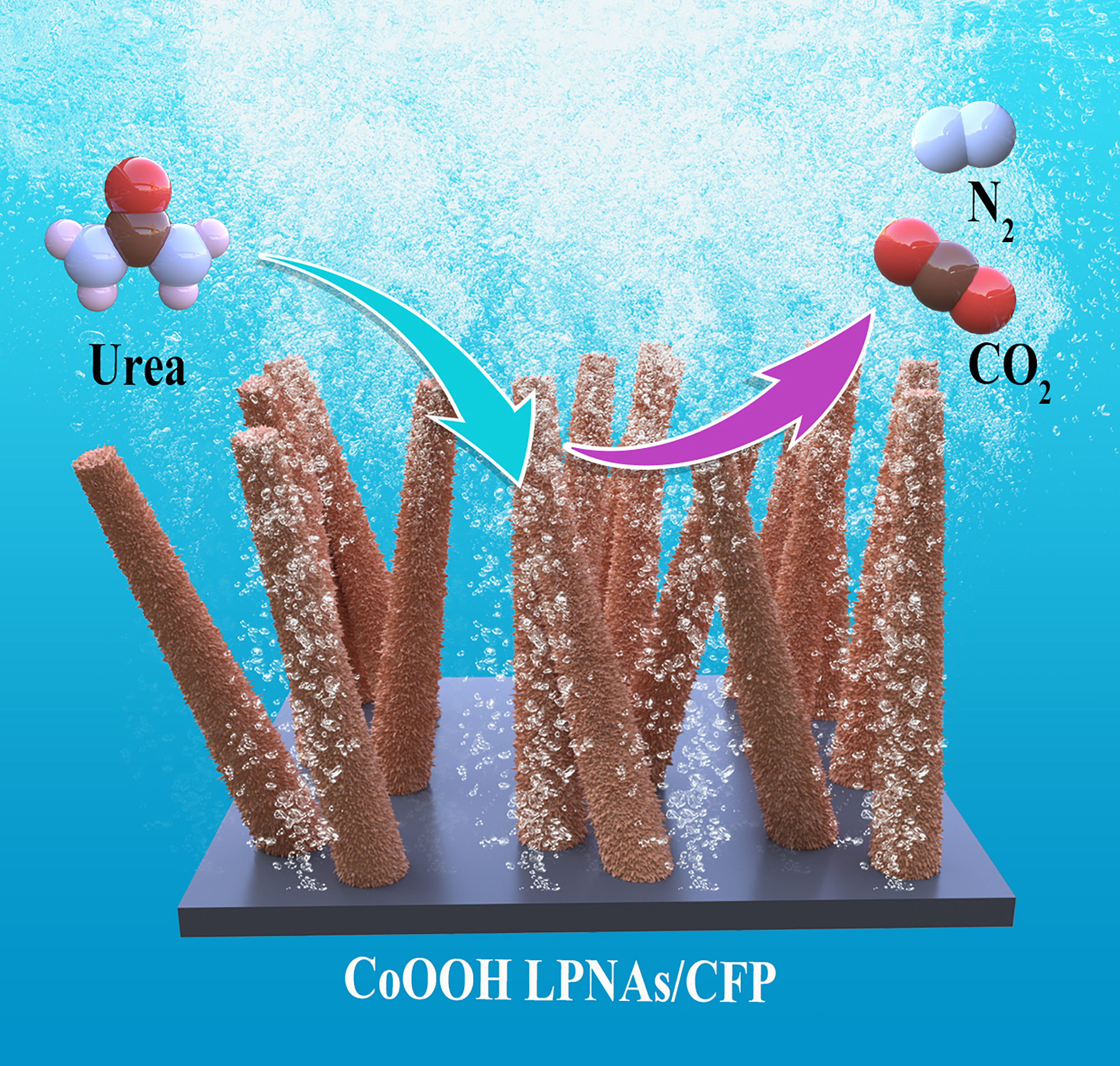
含尿素废水的转化制取清洁氢能技术日益受到关注,但尿素氧化反应(UOR)仍面临催化动力学迟缓和长期稳定性不足的挑战。本研究通过电化学重构CoP纳米针前驱体,在碳纤维纸(CFP)上构建了具有亲水表面和丰富氧空位(Ov)的松散多孔CoOOH纳米结构(CoOOH LPNAs)。该三维电极在1000 mA·cm−2电流密度下展现出1.38 V(vs. RHE)的低电位和优异的UOR耐久性。在阴离子交换膜(AEM)电解槽中,工业级尿素辅助水分解仅需1.53 V即可实现1000 mA·cm−2的电流密度,并稳定运行100小时无衰减。实验与理论研究表明,丰富的氧空位能够有效调控CoOOH的电子结构并且产生Co原子相互靠近的独特Co3三角活性位点。这种协同作用精确调控了反应物及中间产物的吸附-脱附过程,显著降低了UOR的热力学能垒。此外,超亲水自支撑纳米阵列结构促进了反应过程中气体气泡的快速脱附,既提升了整体反应效率,又避免了气泡堆积引发的催化剂剥离问题,从而在高电流密度下同时实现了催化活性和稳定性的双重提升。
吕文静 , 唐小鳗 , 王雪彤 , 刘文才 , 朱鉴文 , 王国静 , 朱远蹠 . 富氧空位的超亲水多孔CoOOH纳米结构用于大电流密度尿素电氧化[J]. 电化学, 2025 , 31(8) : 2503231 . DOI: 10.61558/2993-074X.3550
The conversion of urea-containing wastewater into clean hydrogen energy has gained increasing attention. However, challenges remain, particularly with sluggish catalytic kinetics and limited long-term stability of urea oxidation reaction (UOR). Herein, we report the loosely porous CoOOH nano-architecture (CoOOH LPNAs) with hydrophilic surface and abundant oxygen vacancies (Ov) on carbon fiber paper (CFP) by electrochemical reconstruction of the CoP nanoneedles precursor. The resulting three-dimensional electrode exhibited an impressively low potential of 1.38 V at 1000 mA·cm−2 and excellent durability for UOR. Furthermore, when tested in an anion exchange membrane (AEM) electrolyzer, it required only 1.53 V at 1000 mA·cm−2 for industrial urea-assisted water splitting and operated stably for 100 h without degradation. Experimental and theoretical investigations revealed that rich oxygen vacancies effectively modulate the electronic structure of the CoOOH while creating unique Co3-triangle sites with Co atoms close together. As a result, the adsorption and desorption processes of reactants and intermediates in UOR could be finely tuned, thereby significantly reducing thermodynamic barriers. Additionally, the superhydrophilic self-supported nanoarray structure facilitated rapid gas bubble release, improving the overall efficiency of the reaction and preventing potential catalyst detachment caused by bubble accumulation, thereby improving both catalytic activity and stability at high current densities.

| [1] | Sun H M, Yan Z H, Liu F M, Xu W C, Cheng F Y, Chen J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution[J]. Adv. Mater., 2020, 32(3): e1806326. https://doi.org/10.1002/adma.201806326. |
| [2] | Luo Y T, Zhang Z Y, Chhowalla M, Liu B L. Recent advances in design of electrocatalysts for high-current-density water splitting[J]. Adv. Mater., 2022, 34(16): e2108133. https://doi.org/10.1002/adma.202108133. |
| [3] | Wang G J, Sun Y Z, Zhao Y D, Deng C, Zhu Y Z, Li Y C. Ultrafast electrochemical selenium doping strategy and the role of selenium in nickel-cobalt sulfide for enhanced overall water splitting[J]. Nano Res., 2025, 18(2): 94907165. https://doi.org/10.26599/NR.2025.94907165 |
| [4] | Gong S M, Meng Y, Jin Z Y, Hsu H-Y, Du M S, Liu F. Recent progress on the stability of electrocatalysts under high current densities toward industrial water splitting[J]. ACS Catal., 2024, 14(19): 14399-14435. https://doi.org/10.1021/acscatal.4c03700. |
| [5] | Naik K M, Hashisake K, Higuchi E, Inoue H. Bifunctional intermetallic PdZn nanoparticle-loaded deficient TiO2 nanosheet electrocatalyst for electrochemical water splitting[J]. Mater. Adv., 2023, 4(2): 561-569. https://doi.org/10.1039/D2MA00904H. |
| [6] | Naik K M, Sampath S. Two-step oxygen reduction on spinel NiFe2O4 catalyst: Rechargeable, aqueous solution- and gel-based, Zn-air batteries[J]. Electrochim. Acta, 2018, 292: 268-275. https://doi.org/10.1016/j.electacta.2018.08.138. |
| [7] | Xu S, Ruan X W, Ganesan M, Wu J D, Ravi S K, Cui X Q. Transition metal-based catalysts for urea oxidation reaction (UOR): Catalyst design strategies, applications, and future perspectives[J]. Adv. Funct. Mater., 2024, 34(18): 2313309. https://doi.org/10.1002/adfm.202313309. |
| [8] | Li J X, Wang S L, Sun S J, Wu X, Zhang B G, Feng L G. A review of hetero-structured Ni-based active catalysts for urea electrolysis[J]. J. Mater. Chem. A, 2022, 10(17): 9308-9326. https://doi.org/10.1039/D2TA00120A. |
| [9] | Hu X R, Zhu J Y, Li J F, Wu Q S. Urea electrooxidation: current development and understanding of Ni-based catalysts[J]. ChemElectroChem, 2020, 7(15): 3211-3228. https://doi.org/10.1002/celc.202000404. |
| [10] | Gao X T, Zhang S, Wang P T, Jaroniec M, Zheng Y, Qiao S Z. Urea catalytic oxidation for energy and environmental applications[J]. Chem. Soc. Rev., 2024, 53(3): 1552-1591. https://doi.org/10.1039/D3CS00963G. |
| [11] | Zhang T X, Liu S, Cai W T, He X Y, Wang H Y, Zhu B X, Qin Y, Zhang J, Liu X J, Zhang X, Wang F M. Utilizing cationic vacancies to enhance nickel-cobalt layered double hydroxides for efficient electrocatalytic urea oxidation reaction[J]. Chem. Eng. J., 2024, 500:156766. https://doi.org/10.1016/j.cej.2024.156766. |
| [12] | Zhu B J, Liang Z B, Zou R Q. Designing advanced catalysts for energy conversion based on urea oxidation reaction[J]. Small, 2020, 16(7): e1906133. https://doi.org/10.1002/smll.201906133. |
| [13] | Paygozar S, Sabour Rouh Aghdam A, Hassanizadeh E, Andaveh R, Barati Darband G. Recent progress in non-noble metal-based electrocatalysts for urea-assisted electrochemical hydrogen production[J]. Int. J. Hydrogen Energy, 2023, 48(20): 7219-7259. https://doi.org/10.1016/j.ijhydene.2022.11.087. |
| [14] | Liu H Q, Hu S H, Long B J, Dai H, Yang Y F, Yang M H, Zhang Q, Ke Z, Li W J, He D, Wang Z Y, Xiao X H. In situ unraveling surface reconstruction of Ni-CoP nanowire for excellent alkaline water electrolysis[J]. Energy Environ. Mater., 2024, 8(2): e12834. https://doi.org/10.1002/eem2.12834. |
| [15] | Han X T, Yu C, Niu Y Y, Wang Z, Kang Y B, Ren Y W, Wang H, Park H S, Qiu J. Full bulk-structure reconstruction into amorphorized cobalt-iron oxyhydroxide nanosheet electrocatalysts for greatly improved electrocatalytic activity[J]. Small Methods, 2020, 4(10): 2000546. https://doi.org/10.1002/smtd.202000546. |
| [16] | Liu X, Meng J S, Zhu J X, Huang M, Wen B, Guo R T, Mai L Q. Comprehensive understandings into complete reconstruction of precatalysts: Synthesis, applications, and characterizations[J]. Adv. Mater., 2021, 33(32): e2007344. https://doi.org/10.1002/adma.202007344. |
| [17] | Liu J Z, Guo L. In situ self-reconstruction inducing amorphous species: A key to electrocatalysis[J]. Matter, 2021, 4(9): 2850-2873. https://doi.org/10.1016/j.matt.2021.05.025. |
| [18] | Ye S H, Wang J P, Hu J, Chen Z D, Zheng L R, Fu Y H, Lei Y Q, Ren X Z, He C X, Zhang Q L, Liu J H. Electrochemical construction of low-crystalline CoOOH nanosheets with short-range ordered grains to improve oxygen evolution activity[J]. ACS Catal., 2021, 11(10): 6104-6112. https://doi.org/10.1021/acscatal.1c01300. |
| [19] | Taitt B J, Nam D H, Choi K S. A comparative study of nickel, cobalt, and iron oxyhydroxide anodes for the electrochemical oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid[J]. ACS Catal., 2018, 9(1): 660-670. https://doi.org/10.1021/acscatal.8b04003. |
| [20] | Zhu Y Q, Zhou H, Dong J C, Xu S M, Xu M, Zheng L R, Xu Q, Ma L, Li Z, Shao M F, Duan H H. Identification of active sites formed on cobalt oxyhydroxide in glucose electrooxidation[J]. Angew. Chem. Int. Ed., 2023, 62(15): e202219048. https://doi.org/10.1002/ange.202219048. |
| [21] | Li Z H, Yan Y F, Xu S M, Zhou H, Xu M, Ma L, Shao M F, Kong X G, Wang B, Zheng L R, Duan H H. Alcohols electrooxidation coupled with H2 production at high current densities promoted by a cooperative catalyst[J]. Nat. Commun., 2022, 13(1): 147. https://doi.org/10.1038/s41467-021-27806-3. |
| [22] | Ngo Q P, Prabhakaran S, Kim D H, Kim B S. Rational design of ultrahigh-loading Ir single atoms on reconstructed Mn-NiOOH for enhanced catalytic performance in urea-water electrolysis[J]. Small, 2024, 20(50): e2406786. https://doi.org/10.1002/smll.202406786. |
| [23] | Cai M M, Zhu Q, Wang X Y, Shao Z Y, Yao L, Zeng H, Wu X F, Chen J, Huang K K, Feng S H. Formation and stabilization of NiOOH by introducing α-FeOOH in LDH: Composite electrocatalyst for oxygen evolution and urea oxidation reactions[J]. Adv. Mater., 2023, 35(7): e2209338. https://doi.org/10.1002/adma.202209338. |
| [24] | Mariappan A, Mannu P, Ranjith K S, Nga T T T, Han Y K, Dong C L, Dharman R K, Oh T H. Novel heterostructure-based CoFe and cobalt oxysulfide nanocubes for effective bifunctional electrocatalytic water and urea oxidation[J]. Small, 2024, 20(26): 2310112. https://doi.org/10.1002/smll.202310112. |
| [25] | Guo X, Qiu L Y, Li M G, Tian F Y, Ren X, Jie S, Geng S, Han G H, Huang Y R, Song Y, Yang W W, Yu Y S. Accelerating the generation of NiOOH by in-situ surface phosphating nickel sulfide for promoting the proton-coupled electron transfer kinetics of urea electrolysis[J]. Chem. Eng. J., 2024, 483: 149264. https://doi.org/10.1016/j.cej.2024.149264. |
| [26] | Song S Z, Bao H L, Lin X, Du X L, Zhou J, Zhang L J, Chen N, Hu J, Wang J Q. Molten salt-assisted synthesis of bulk CoOOH as a water oxidation catalyst[J]. J. Energy Chem., 2020, 42: 5-10. https://doi.org/10.1016/j.jechem.2019.05.021. |
| [27] | Lv J Q, Guan X F, Huang Y Y, Cai L X, Yu M X, Li X Y, Yu Y L, Chen D G. Stepwise chemical oxidation to access ultrathin metal (oxy)-hydroxide nanosheets for the oxygen evolution reaction[J]. Nanoscale, 2021, 13(37): 15755-15762. https://doi.org/10.1039/D1NR03813C. |
| [28] | Yang H Y, Driess M, Menezes P W. Self-supported electrocatalysts for practical water electrolysis[J]. Adv. Energy Mater., 2021, 11(39): 2102074. https://doi.org/10.1002/aenm.202102074. |
| [29] | Iwata R, Zhang L, Wilke K L, Gong S, He M, Gallant B M, Wang E N. Bubble growth and departure modes on wettable/non-wettable porous foams in alkaline water splitting[J]. Joule, 2021, 5(4): 887-900. https://doi.org/10.1016/j.joule.2021.02.015. |
| [30] | Qin H Y, Ye Y K, Li J H, Jia W Q, Zheng S Y, Cao X J, Lin G L, Jiao L F. Synergistic engineering of doping and vacancy in Ni(OH)2 to boost urea electrooxidation[J]. Adv. Funct. Mater., 2022, 33(4): 2209698. https://doi.org/10.1002/adfm.202209698. |
| [31] | Yan M L, Zhang J J, Wang C, Gao L, Liu W G, Zhang J H, Liu C Q, Lu Z W, Yang L J, Jiang C L, Zhao Y. Synergistic engineering of heterostructure and oxygen vacancy in cobalt hydroxide/aluminum oxyhydroxide as bifunctional electrocatalysts for urea-assisted hydrogen production[J]. J. Colloid Interface Sci., 2025, 677(Pt A): 1069-1079. https://doi.org/10.1016/j.jcis.2024.07.239. |
| [32] | Li L F, Zhang X, Humayun M, Xu X F, Shang Z X, Li Z S, Yuen M F, Hong C X, Chen Z H, Zeng J R, Bououdina M, Temst K, Wang X, Wang C. Manipulation of electron spins with oxygen vacancy on amorphous/crystalline composite-type catalyst[J]. ACS Nano, 2024, 18(1): 1214-1225. https://doi.org/10.1021/acsnano.3c12133. |
| [33] | Tong Y, Chen P Z, Zhang M X, Zhou T P, Zhang L D, Chu W S, Wu C Z, Xie Y. Oxygen vacancies confined in nickel molybdenum oxide porous nanosheets for promoted electrocatalytic urea oxidation[J]. ACS Catal., 2017, 8(1): 1-7. https://doi.org/10.1021/acscatal.7b03177. |
| [34] | Huang B, Wang J N, Xie D L, Huang Q P, Wen D, Zeng X Q, Lin D M, Guo W H, Sun H C, Xie F Y. Surface reconstruction of defect-engineered MIL-88@Fe2O3 p-n heterojunction for enhanced electrocatalytic water and urea oxidation[J]. Chem. Eng. J., 2024, 498:155006. https://doi.org/10.1016/j.cej.2024.155006. |
| [35] | Xu L, Jiang Q Q, Xiao Z H, Li X Y, Huo J, Wang S Y, Dai L M. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction[J]. Angew. Chem. Int. Ed., 2016, 55(17): 5277-5281. https://doi.org/10.1002/anie.201600687. |
| [36] | Zhang B B, Huang X J, Hu H Y, Chou L J, Bi Y P. Defect-rich and ultrathin CoOOH nanolayers as highly efficient oxygen evolution catalysts for photoelectrochemical water splitting[J]. J. Mater. Chem. A, 2019, 7(9): 4415-4419. https://doi.org/10.1039/C8TA12012A. |
| [37] | Ou Y Q, Tian W Q, Liu L, Zhang Y H, Xiao P. Bimetallic Co2Mo3O8 suboxides coupled with conductive cobalt nanowires for efficient and durable hydrogen evolution in alkaline electrolyte[J]. J. Mater. Chem. A, 2018, 6(12): 5217-5228. https://doi.org/10.1039/C7TA11401J. |
| [38] | Cho I S, Logar M, Lee C H, Cai L, Prinz F B, Zheng X. Rapid and controllable flame reduction of TiO2 nanowires for enhanced solar water-splitting[J]. Nano Lett., 2014, 14(1): 24-31. https://doi.org/10.1021/nl4026902. |
| [39] | Kresse G, Furthmuller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Phys. Rev. B, 1996, 54(16): 11169-11186. https://doi.org/10.1103/PhysRevB.54.11169. |
| [40] | Hafner J. Ab-initio simulations of materials using vasp: Density-functional theory and beyond[J]. J. Comput. Chem., 2008, 29(13): 2044-2078. https://doi.org/10.1002/jcc.21057. |
| [41] | Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation[J]. Phys. Rev. B, 1992, 46(11): 6671-6687. https://doi.org/10.1103/PhysRevB.46.6671. |
| [42] | Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Phys. Rev. Lett., 1996, 77(18): 3865-3868. https://doi.org/10.1103/PhysRevLett.77.3865. |
| [43] | Rossmeisl J, Logadottir A, Norskov J K. Electrolysis of water on (oxidized) metal surfaces[J]. Chem. Phys., 2005, 319(1-3): 178-184. https://doi.org/10.1016/j.chemphys.2005.05.038. |
| [44] | Peterson A A, Abild-Pedersen F, Studt F, Rossmeisl J, Norskov J K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels[J]. Energy Environ. Sci., 2010, 3(9): 1311-1315. https://doi.org/10.1039/C0EE00071J. |
| [45] | Ji L L, Wang J Y, Teng X, Meyer T J, Chen Z. CoP nanoframes as bifunctional electrocatalysts for efficient overall water splitting[J]. ACS Catal., 2019, 10(1): 412-419. https://doi.org/10.1021/acscatal.9b03623. |
| [46] | Fan K, Zou H Y, Lu Y, Chen H, Li F S, Liu J X, Sun L D, Tong L P, Toney M F, Sui M L, Yu J G. Direct observation of structural evolution of metal chalcogenide in electrocatalytic water oxidation[J]. ACS Nano, 2018, 12(12): 12369-12379. https://doi.org/10.1021/acsnano.8b06312. |
| [47] | Xu S, Jiao D X, Ruan X W, Jin Z Y, Qiu Y, Feng Z P, Zheng L R, Fan J C, Zheng W T, Cui X Q. O-2p hybridization enhanced transformation of active γ-NiOOH by chromium doping for efficient urea oxidation reaction[J]. Adv. Funct. Mater., 2024, 34(36): 2401265. https://doi.org/10.1002/adfm.202401265. |
| [48] | Chen Z Y, Song Y, Cai J Y, Zheng X S, Han D D, Wu Y S, Zang Y P, Niu S W, Liu Y F, Zhu J F, Liu X J, Wang G M. Tailoring the d-band centers enables Co4N nanosheets to be highly active for hydrogen evolution catalysis[J]. Angew. Chem. Int. Ed., 2018, 57(18): 5076-5080. https://doi.org/10.1002/anie.201801834. |
| [49] | Chen H Y, Xu Y S, Li X J, Ma Q, Xie D L, Mei Y, Wang G J, Zhu Y Z. Hierarchical NiCo2Se4 arrays composed of atomically thin nanosheets: Simultaneous improvements in thermodynamics and kinetics for electrocatalytic water splitting in neutral media[J]. Adv. Sci., 2024, 11(31): 2402889. https://doi.org/10.1002/advs.202402889. |
| [50] | Hammer B, Norskov J K. Why gold is the noblest of all the metals[J]. Nature, 1995, 376(6537): 238-240. https://doi.org/10.1038/376238a0. |
/
| 〈 |
|
〉 |