

Fe3C纳米晶体电催化一氧化氮还原合成氨
收稿日期: 2024-12-17
修回日期: 2025-01-30
录用日期: 2025-02-08
网络出版日期: 2025-02-15
Electrocatalytic Nitric Oxide Reduction to Yield Ammonia over Fe3C Nanocrystals
Received date: 2024-12-17
Revised date: 2025-01-30
Accepted date: 2025-02-08
Online published: 2025-02-15
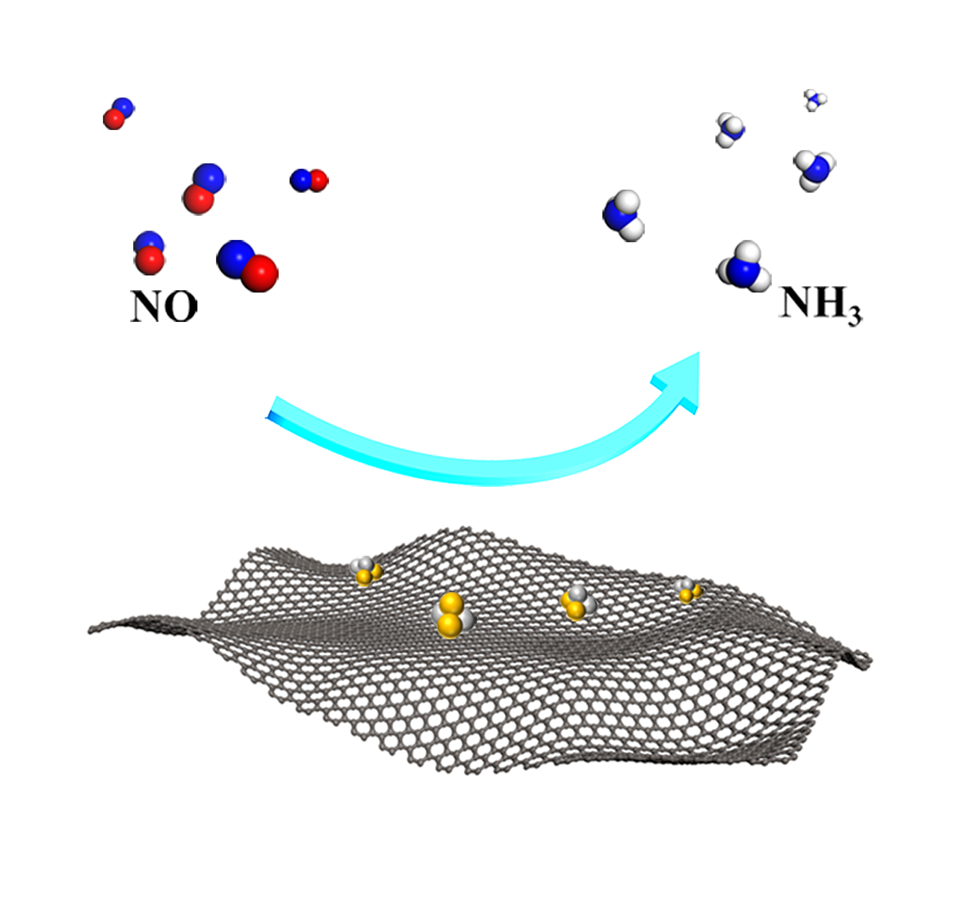
一氧化氮(NO)通常来自汽车尾气和工业烟气,是最严重的空气污染物之一。为解决这一问题,电催化一氧化氮还原反应(NORR)被提出,其不仅能去除NO大气污染物,还能产生有价值的氨(NH3)。因此,通过对Fe3C纳米晶体进行改性,制备的Fe3C/C-900样品在常温常压条件下可作为高效NORR催化剂。电化学测试结果表明,Fe3C/C-900催化剂在-0.8 V(vs. RHE)时的合成NH3法拉第效率为76.5%,在-1.2 V(vs. RHE)时的NH3产率为177.5 μmol·h-1·cm-2。同时,其在长时间电解过程中,也能保持稳定的NORR活性。此外,我们将Fe3C/C-900的高NORR特性归因于两个方面:一方面,Fe3C纳米晶体的内在活性,包括降低决速步骤(*NOH→*N)能垒和抑制氢析出副反应;另一方面,多孔碳基底有利于活性成分的分散、气态NO反应物的有效吸附和液态NH3产物的释放。
林森 , 张浪 , 侯童 , 丁俊阳 , 彭紫默 , 刘亦帆 , 刘熙俊 . Fe3C纳米晶体电催化一氧化氮还原合成氨[J]. 电化学, 2025 , 31(4) : 2412171 . DOI: 10.61558/2993-074X.3525
Nitric oxide (NO), which generally originates from vehicle exhaust and industrial flue gases, is one of the most serious air pollutants. In this case, the electrochemical NO reduction reaction (NORR) not only removes the atmospheric pollutant NO but also produces valuable ammonia (NH3). Hence, through the synthesis and modification of Fe3C nanocrystal catalysts, the as-obtained optimal sample of Fe3C/C-900 was adopted as the NORR catalyst at ambient conditions. As a result, the Fe3C/C-900 catalyst showed an NH3 Faraday efficiency of 76.5% and an NH3 yield rate of 177.5 μmol·h-1·cm-2 at the working potentials of -0.8 and -1.2 V versus reversible hydrogen electrode (vs. RHE), respectively. And it delivered a stable NORR activity during the electrolysis. Moreover, we attribute the high NORR properties of Fe3C/C-900 to two aspects: one is the enhanced intrinsic activity of Fe3C nanocrystals, including the lowering of the energy barrier of rate-limiting step (*NOH→*N) and the inhibition of hydrogen evolution; on the other hand, the favorable dispersion of active components, the effective adsorption of gaseous NO, and the release of liquid NH3 products facilitated by the porous carbon substrate.

| [1] | Almaraz M, Bai E, Wang C, Trousdell J, Conley S, Faloona I, Houlton B Z. Agriculture is a major source of NOx pollution in California[J]. Sci. Adv., 2024, 4(1): eaao3477. |
| [2] | Fernandes C, Holz L I V, Loureiro F J A, Duarte M, Fagg D P, Mendes A. Nitrous oxide abatement and valorization in an ammonia-fueled SOFC stack - Nitric oxide and electricity production[J]. Chem. Eng. J., 2024, 502: 157921. |
| [3] | Alves L, Holz L I V, Fernandes C, Ribeirinha P, Mendes D, Fagg D P, Mendes A. A comprehensive review of NOx and N2O mitigation from industrial streams[J]. Renew. Sust. Energ. Rev., 2022, 155: 111916. |
| [4] | Kim H S, Kasipandi S, Kim J, Kang S H, Kim J H, Ryu J H, Bae J W. Current catalyst technology of selective catalytic reduction (SCR) for NOx removal in South Korea[J]. Catalysts, 2020, 10(1): 52. |
| [5] | Nittoor-Veedu R, Ju X, Pumera M. Iron single atom catalysts for electrochemical ammonia synthesis: toward carbon free hydrogen storage[J]. Adv. Energy Mater., 2024: DOI:10.1002/aenm.202402205. |
| [6] | Ouyang L, Liang J, Luo Y S, Zheng D D, Sun S J, Liu Q, Hamdy M S, Sun X P, Ying B W. Recent advances in electrocatalytic ammonia synthesis[J]. Chin. J. Catal., 2023, 50: 6-44. |
| [7] | Liang J, Chen H Y, Mou T, Zhang L C, Lin Y T, Yue L C, Luo Y S, Liu Q, Li N, Alshehri A A, Shakir I, Agboola P O, Wang Y Y, Tang B, Ma D W, Sun X P. Coupling denitrification and ammonia synthesis via selective electrochemical reduction of nitric oxide over Fe2O3 nanorods[J]. J. Mater. Chem. A, 2022, 10(12): 6454-6462. |
| [8] | Cheon S, Kim W J, Kim D Y, Kwon Y, Han J I. Electro-synthesis of ammonia from dilute nitric oxide on a gas diffusion electrode[J]. ACS Energy Lett., 2022, 7(3): 958-965. |
| [9] | Zhao S W, Chang M W, Liu J Y, Shi G S, Yang Y Q, Gu H L, Zhang J H, Yang C L, Tong H N, Zhu C Y, Cao K C, Li S Z, Zhang L M. Selective nitric oxide electroreduction at monodispersed transition-metal sites with atomically precise coordination environment[J]. Chem. Catal., 2023, 3(6): 100598. |
| [10] | Qian S J, Cao H, Wang Y G, Li J. Controlling the selectivity of electrocatalytic NO Reduction through pH and potential regulation on single-atom catalysts[J]. J. Am. Chem. Soc., 2024, 146(18): 12530-12537. |
| [11] | Shao J Q, Jing H J, Wei P F, Fu X Y, Pang L, Song Y P, Ye K, Li M R, Jiang L Z, Ma J Y, Li R T, Si R, Peng Z Q, Wang G X, Xiao J P. Electrochemical synthesis of ammonia from nitric oxide using a copper-tin alloy catalyst[J]. Nat. Energy, 2023, 8(11): 1273-1283. |
| [12] | Chai L L, Zhang L J, Wang X, Xu L Q, Han C, Li T T, Hu Y, Qian J J, Huang S M. Bottom-up synthesis of MOF-derived hollow N-doped carbon materials for enhanced ORR performance[J]. Carbon, 2019, 146: 248-256. |
| [13] | Yang J P, Zhang F Z, Chen J. Structural design and application of fiber-based electrocatalytic materials[J]. China Powder Sci. Technol., 2024, 30(4): 161-170. |
| [14] | Qin P G, Han L Z, Zhang X W, Li M Y, Li D, Lu M H, Cai Z W. MIL-101(Fe)-derived magnetic porous carbon as sorbent for stir bar sorptive-dispersive microextraction of sulfonamides[J]. Microchim. Acta, 2021, 188(10): 340. |
| [15] | Hu X, Li Y, Zeng G, Jia J C, Zhan H B, Wen Z H. Three-dimensional network architecture with hybrid nanocarbon composites supporting few-layer MoS2 for lithium and sodium storage[J]. ACS Nano, 2018, 12(2): 1592-1602. |
| [16] | Alshehri S M, Alhabarah A N, Ahmed J, Naushad M, Ahamad T. An efficient and cost-effective tri-functional electrocatalyst based on cobalt ferrite embedded nitrogen doped carbon[J]. J. Colloid Interface Sci., 2018, 514: 1-9. |
| [17] | Iriawan H, Andersen S Z, Zhang X, Comer B M, Barrio J, Chen P, Medford A J, Stephens I E L, Chorkendorff I, Shao-Horn Y. Methods for nitrogen activation by reduction and oxidation[J]. Nat. Rev. Methods Primers, 2021, 1(1): 56. |
| [18] | Greczynski G, Hultman L. The same chemical state of carbon gives rise to two peaks in X-ray photoelectron spectroscopy[J]. Sci. Rep., 2021, 11(1): 11195. |
| [19] | Wu K S, Hu Y, Cheng Z L, Pan P, Zhang M M, Jiang L Y, Mao J T, Ni C K, Zhang Y R, Wang Z X, Gu X F, Zhang X W. Fe3C composite carbon nanofiber interlayer for efficient trapping and conversion of polysulfides in lithium-sulfur batteries[J]. J. Alloys Compd., 2020, 847: 156443. |
| [20] | Liu X J, Chen M Y, Ma J J, Liang J Q, Li C S, Chen C J, He H B. Advances in the synthesis strategies of carbon-based single-atom catalysts and their electrochemical applications[J]. China Powder Sci. Technol., 2024, 30: 35-45. |
| [21] | Li Z J, Ma Q, Li Z Z, Zhang D, Sun Q J, Wang Q J, Sun H L, Wang B. Exploring the role of solvents in structural regulation during ultrasonic synthesis of Co/Ni-layered double hydroxide for oxygen evolution reaction[J]. Mater. Rep. Energy, 2024, 4(4): 100296. |
| [22] | Wu H G, Fei G T, Gao X D, Guo X, Gong X X, Ma X L, Wang Q, Xu S H. Research progress on preparation and application of polyaniline and its composite materials[J]. China Powder Sci. Technol., 2023, 29(5): 70-80. |
| [23] | Ma X L, Xu C G, Yang Y, Sun D, Zhao K, Lu C B, Jin P, Chong Y T, Pruksawan S, Xiao Z H, Wang F K. S-doped mesoporous graphene modified separator for high performance lithium-sulfur batteries[J]. Mater. Rep. Energy, 2024, 4(3): 100279. |
| [24] | Bl?chl P E. Projector augmented-wave method[J]. Phys. Rev. B, 1994, 50(24): 17953-17979. |
| [25] | Wang D D, Fan G L, Luan D Y, Guo Y, Gu X J, Lou X W. Ru-incorporation-induced phase transition in Co nanoparticles for low-concentration nitric oxide electroreduction to ammonia at low potential[J]. Adv. Mater., 2024, 36(50): 2408580. |
| [26] | Jamadar A S, Sutar R, Patil S, Khandekar R, Yadav J B. Progress in metal oxide-based electrocatalysts for sustainable water splitting[J]. Mater. Rep. Energy, 2024, 4(3): 100283. |
| [27] | Zhang H F, Li Y B, Cheng C Q, Zhou J, Yin P F, Wu H M, Liang Z Q, Zhang J W, Yun Q B, Wang A L, Zhu L J, Zhang B, Cao W B, Meng X M, Xia J, Yu Y F, Lu Q P. Isolated electron-rich ruthenium atoms in intermetallic compounds for boosting electrochemical nitric oxide reduction to ammonia[J]. Angew. Chem. Int. Ed., 2023, 62(4): e202213351. |
| [28] | Ma J B, Lin S, Lin Z Q, Sun L, Lin C J. Recent advances in solar photo(electro)catalytic nitrogen fixation[J]. J. Electrochem., 2024, 30(3): 2314003. |
| [29] | Cui Y H, Sun C N, Ding G P, Zhao M, Ge X, Zhang W, Zhu Y F, Wang Z L, Jiang Q. Synergistically tuning intermediate adsorption and promoting water dissociation to facilitate electrocatalytic nitrate reduction to ammonia over nanoporous Ru-doped Cu catalyst[J]. Sci. China Mater., 2023, 66(11): 4387-4395. |
| [30] | Yang M S, Wei T R, He J, Liu Q, Feng L G, Li H Y, Luo J, Liu X J. Au nanoclusters anchored on TiO2 nanosheets for high-efficiency electroreduction of nitrate to ammonia[J]. Nano Res., 2024, 17(3): 1209-1216. |
| [31] | Sun H, Kim H, Song S Z, Jung W. Copper foam-derived electrodes as efficient electrocatalysts for conventional and hybrid water electrolysis[J]. Mater. Rep. Energy, 2022, 2(2): 100092. |
| [32] | Zhang P F, Hong S H, Song N, Han Z H, Ge F, Dai G, Dong H J, Li C M. Alloy as advanced catalysts for electrocatalysis: From materials design to applications[J]. Chin. Chem. Lett., 2024, 35(6): 109073. |
/
| 〈 |
|
〉 |