

无空气环境下平管型SOEC电解堆CO2/H2O共电解稳定性研究
收稿日期: 2024-11-12
修回日期: 2024-12-20
录用日期: 2025-01-06
网络出版日期: 2025-01-08
Performance of CO2/H2O Co-Electrolysis in a Flat-Tube Solid Oxide Electrolysis Cell Stack under an Air-Free Environment
Received date: 2024-11-12
Revised date: 2024-12-20
Accepted date: 2025-01-06
Online published: 2025-01-08
钟小慧 , 王飞 , 武安祺 , 韩贝贝 , 王建新 , 官万兵 . 无空气环境下平管型SOEC电解堆CO2/H2O共电解稳定性研究[J]. 电化学, 2025 , 31(4) : 2411121 . DOI: 10.61558/2993-074X.3518
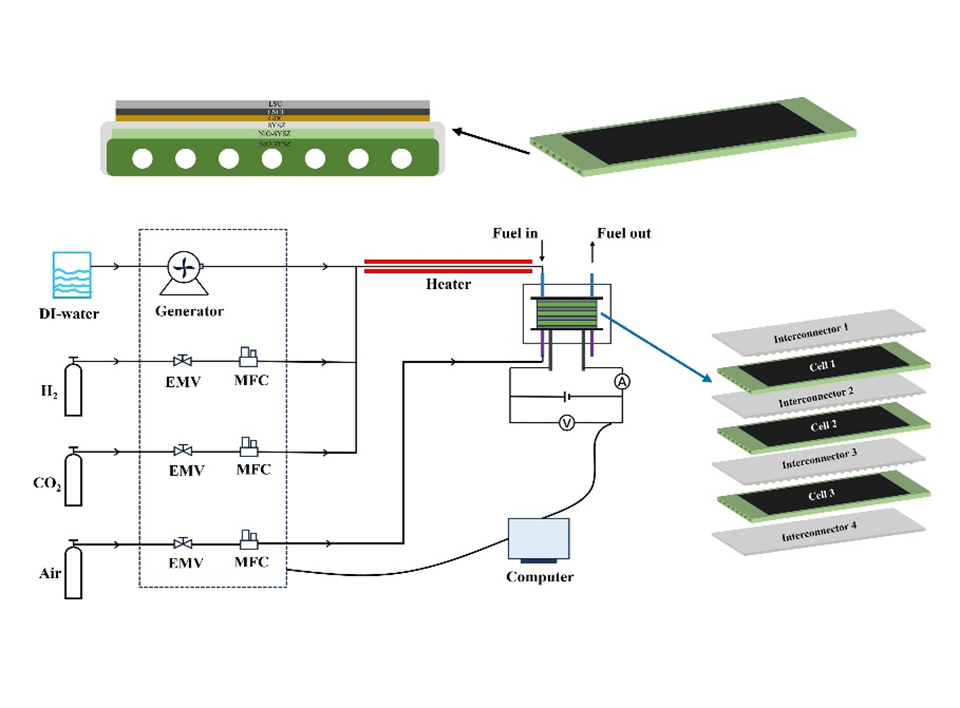
This work investigates the transient performance and stability of CO2/H2O co-electrolysis in an air-free environment using a flat-tube solid oxide electrolysis cell (SOEC) stack. The results showed that the transient behavior of the stack with and without blowing gas into the air electrode is almost the same. With a current density of 0.67A·cm-2 @750 °C, the stack operated for over 200 h under co-electrolysis conditions without air blowing, and the voltage drop rate of the stack was approximately 0.203%/100 hours. Microstructure analyses revealed a significant loss of nickel particles and an apparent formation of an insulating phase strontium chromate (SrCrO4) on the surface of the current collection layer of the air electrode, which are identified as key factors contributing to the performance degradation of the stack. This study provides a reference for development of efficient fuel preparation technology based on SOEC stack in airless environments.
| [1] | Dybkjaer I, Aasberg-Petersen K. Synthesis gas technology large‐scale applications[J]. Can. J. Chem. Eng., 2016, 94(4): 607-612. |
| [2] | Kousheshi N, Yari M, Paykani A, Mehr A, Fuente G. Effect of syngas composition on the combustion and emissions characteristics of a syngas/diesel RCCI engine[J]. Energies, 2020, 13(1): 212. |
| [3] | Shahbaz M, Yusup S, Inayat A, Patrick D, Pratama A. Application of response surface methodology to investigate the effect of different variables on conversion of palm kernel shell in steam gasification using coal bottom ash[J]. Appl. Energy, 2016, 184: 1306-1315. |
| [4] | Yoon S J, Lee J. Hydrogen-rich syngas production through coal and charcoal gasification using microwave steam and air plasma torch[J]. Int. J. Hydrogen Energy, 2012, 37(22): 17093-17100. |
| [5] | Szczygie? J, Chojnacka K, Skrzypczak D, Izydorczyk G, Moustakas K, Ku?a?yński M. Using greenhouse gases in the synthesis gas production processes: Thermodynamic conditions[J]. J. Environ. Manag., 2022, 325(A): 116463. |
| [6] | Yentekakis I V, Panagiotopoulou P, Artemakis G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations[J]. Appl. Catal. B Environ. Energy, 2021, 296: 120210. |
| [7] | Zhao R, Cao K, Ye R L, Tang Y T, Du C, Liu F, Zhao Y K, Chen R, Shan B. Deciphering the stability mechanism of Pt-Ni/Al2O3 catalysts in syngas production via DRM[J]. Chem. Eng. J., 2024, 491: 151966. |
| [8] | Chen X B, Guan C Z, Xiao G P, Du X L, Wang J Q. Syngas production by high temperature steam/CO coelectrolysis using solid oxide electrolysis cells[J]. Faraday Discuss., 2015, 182: 329-339. |
| [9] | Cui C S, Wang Y, Tong Y C, Wang S W, Chen C S, Zhan Z L. Syngas production through CH4-assisted co-electrolysis of H2O and CO2 in La0.8Sr0.2Cr0.5Fe0.5O3-δ-Zr0.84Y0.16O2-δ electrode-supported solid oxide electrolysis cells[J]. Int. J. Hydrogen Energy, 2021, 46(39): 20305-20312. |
| [10] | Fei Y X, Li A, Zhang C, Tu H Y, Zhu L, Huang Z. Performance optimization of solid oxide electrolysis cell for syngas production by high temperature co-electrolysis via differential evolution algorithm with practical constraints[J]. Energy Convers. Manag., 2023, 300: 117911. |
| [11] | Li Z, Zhang H, Xu H R, Xuan J. Advancing the multiscale understanding on solid oxide electrolysis cells via modelling approaches: A review[J]. Renew. Sust. Energ. Rev., 2021, 141: 110863. |
| [12] | Zhang B Z, Zhang Z, Zhang L J, Tang K B, Xia C R. A perovskite infiltrated cathode of metal-supported solid oxide electrolysis cell for CO2 electrolysis[J]. Int. J. Hydrogen Energy, 2023, 49(C): 417-423. |
| [13] | Zheng Y F, Zhou J, Zhang L, Liu Q L, Pan Z H, Chan S. High-temperature electrolysis of simulated flue gas in solid oxide electrolysis cells[J]. Electrochim. Acta, 2018, 280: 206-215. |
| [14] | Guan W B, Du Z G, Wang J X, Jiang L, Yang J, Zhou X D. Mechanisms of performance degradation induced by thermal cycling in solid oxide fuel cell stacks with flat-tube anode-supported cells based on double-sided cathodes[J]. Int. J. Hydrogen Energy, 2020, 45(38): 19840-19846. |
| [15] | Sun X F, Hendriksen P, Hauch A, Clausen A, Lehtinen T, Noponen M. Effect of anode side purge gas on the degradation of solid oxide electrolysis cells[J]. ECS Trans., 2021, 103: 1083. |
| [16] | Liu Z, Han B B, Lu Z Y, Guan W B, Li Y Y, Song C J, Chen L, Singhal S. Efficiency and stability of hydrogen production from seawater using solid oxide electrolysis cells[J]. Appl. Energy. 2021, 300: 117439. |
| [17] | Song Z H, Pan H, Wan G C, Wu A Q, Chen Q J, Guan W B, Singhal S. Enhancing durability of solid oxide cells for hydrogen production from seawater by designing nano-structured Sm0.5Sr0.5Co3-δ infiltrated air electrodes[J]. Int. J. Hydrogen Energy, 2023, 48(70): 27095-27104. |
| [18] | Hu X G, Yang Y P, Han B B, Huang X R, Lei J Y, Sang J K, Wu A Q, Liu Z, Lu Z Y, Guan W B. Efficiency and stability of seawater electrolysis through flat-tube solid oxide cell stack without air[J]. Int. J. Hydrogen Energy, 2023, 55: 909-916. |
| [19] | Liang J J, Han M F. Different performance and mechanisms of CO2 electrolysis with CO and H2 as protective gases in solid oxide electrolysis cell[J]. Int. J. Hydrogen Energy, 2022, 47(43): 18606-18618. |
| [20] | Stempien J, Liu Q L, Ni M, Sun Q, Chan S. Physical principles for the calculation of equilibrium potential for co-electrolysis of steam and carbon dioxide in a solid oxide electrolyzer cell (SOEC)[J]. Electrochim. Acta, 2014, 147: 490-497. |
| [21] | Xiong M, Han B B, Yao Y, Wu A Q, Gao Y F, Guan W B. Effect of seawater on the performance of flat-tube solid oxide cell for CO2/H2O co-electrolysis[J]. Fuel, 2023, 357(C): 130039. |
| [22] | Xi C Q, Sang J K, Wu A Q, Yang J, Qi X P, Guan W B, Wang J X, Singhal S. Electrochemical performance and durability of flat-tube solid oxide electrolysis cells for H2O/CO2 co-electrolysis[J]. Int. J. Hydrogen Energy, 2022, 47(18): 10166-10174. |
| [23] | Rao M, Sun X, Hagen A. A comparative study of durability of solid oxide electrolysis cells tested for Co-electrolysis under galvanostatic and potentiostatic conditions[J]. J. Electrochem. Soc., 2018, 165(10): F748-F755. |
| [24] | Tao Y K, Ebbesen S, Mogensen M. Degradation of solid oxide cells during co-electrolysis of steam and carbon dioxide at high current densities[J]. J. Power Sources, 2016, 328: 452-462. |
| [25] | Sun X, Hendriksen P, Mogensen M, Chen M. Degradation in solid oxide electrolysis cells during long term testing[J]. Fuel Cells, 2019, 19(6): 740-747. |
| [26] | Li C L, Wu A Q, Xi C Q, Guan W B, Chen L, Singhal S. High reversible cycling performance of carbon dioxide electrolysis by flat-tube solid oxide cell[J]. Appl. Energy, 2022, 314: 118969. |
| [27] | Hu B X, Krishnan S, Liang C Y, Heo S, Aphale A, Ramprasad R, Singh P. Experimental and thermodynamic evaluation of La1-xSrxMnO3±δ and La1-xSrxCo1-yFeyO3-δ cathodes in Cr-containing humidified air[J]. Int. J. Hydrogen Energy, 2017, 42(15): 10208-10216. |
| [28] | Abernathy H, Koep E, Compson C, Cheng Z, Liu M L. Monitoring Ag-Cr interactions in SOFC cathodes using Raman spectroscopy[J]. J. Phys. Chem. C., 2008, 112(34): 13299-13303. |
/
| 〈 |
|
〉 |