

铜取代型Li2Ni1-xCuxO2正极补锂剂提升石墨/磷酸铁锂电池循环寿命
收稿日期: 2024-08-29
修回日期: 2024-11-25
录用日期: 2024-11-26
网络出版日期: 2024-12-13
Enhancing Cycle Life of Graphite‖LiFePO4 Batteries via Copper Substituted Li2Ni1-xCuxO2 Cathode Prelithiation Additive
Received date: 2024-08-29
Revised date: 2024-11-25
Accepted date: 2024-11-26
Online published: 2024-12-13
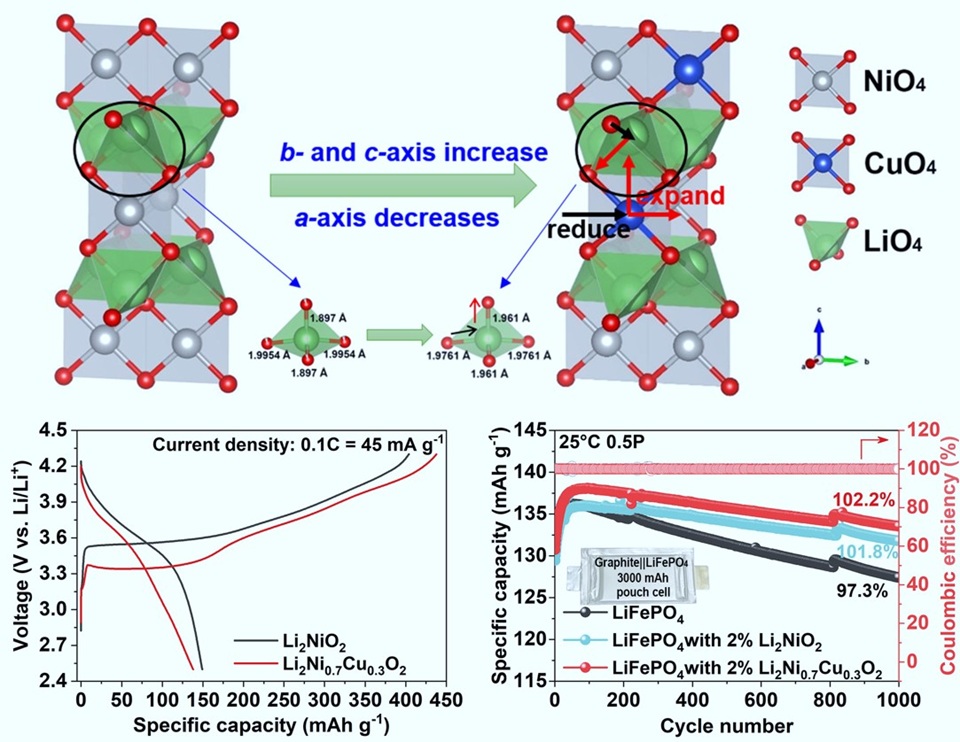
镍酸锂(Li2NiO2)作为一种正极补锂剂,用于补偿锂离子损失,以提高锂离子电池的循环寿命。然而,Li2NiO2补锂剂存在成本高、脱锂动力学较差的问题。本文研究了基于高温固相法合成的低成本铜(Cu)取代型Li2Ni1-xCuxO2(x = 0, 0.2, 0.3, 0.5, 0.7)补锂剂对石墨/磷酸铁锂电池结构、形貌和电化学性能的影响。晶体结构精修结果表明,Cu取代策略有利于消除NiOx杂质相,弱化Li-O键。理论计算结果显示Cu取代可提高补锂剂电子电导率。同时,电化学分析证实Cu取代有利于降低脱锂电位,提高脱锂容量。其中,最优比例的Li2Ni0.7Cu0.3O2具有437 mAh·g-1的高脱锂容量,较Li2NiO2提升约8%。此外,设计了容量为3000 mAh的石墨/磷酸铁锂软包电池,采用2 wt% Li2Ni0.7Cu0.3O2补锂剂,其1000次循环的可逆容量、能量密度和循环寿命显著提高;其首圈放电容量提升6.2 mAh·g-1,0.5 P循环1000圈容量保持率提升约5%。对循环后的电池进行拆解分析后发现Li2Ni0.7Cu0.3O2添加剂可以减少固体电解质界面(SEI)的分解、改善脱嵌锂的均一性,从而提高石墨负极与电解液之间的界面稳定性,抑制析锂。综上,与Li2NiO2相比,Li2Ni0.7Cu0.3O2具有成本低、脱锂电压低和预锂化容量高的优点,是极具前景的下一代锂离子电池正极补锂剂材料。
关键词: Li2Ni1-xCuxO2; 正极补锂剂; 磷酸铁锂电池; 循环寿命; 电网储能
郑建明 , 张静文 , 焦天鹏 . 铜取代型Li2Ni1-xCuxO2正极补锂剂提升石墨/磷酸铁锂电池循环寿命[J]. 电化学, 2025 , 31(2) : 2408301 . DOI: 10.61558/2993-074X.3515
Lithium nickel oxide (Li2NiO2), as a sacrificial cathode prelithiation additive, has been used to compensate for the lithium loss for improving the lifespan of lithium-ion batteries (LIBs). However, high-cost Li2NiO2 suffers from inferior delithiation kinetics during the first cycle. Herein, we investigated the effects of the cost-effective copper substituted Li2Ni1-xCuxO2 (x = 0, 0.2, 0.3, 0.5, 0.7) synthesized by a high-temperature solid-phase method on the structure, morphology, electrochemical performance of graphite‖LiFePO4 battery. The X-ray diffraction (XRD) refinement result demonstrated that Cu substitution strategy could be favorable for eliminating the NiOx impurity phase and weakening Li-O bond. Analysis on density of states (DOS) indicates that Cu substitution is good for enhancing the electronic conductivity, as well as reducing the delithiation voltage polarization confirmed by electrochemical characterizations. Therefore, the optimal Li2Ni0.7Cu0.3O2 delivered a high delithiation capacity of 437 mAh·g-1, around 8% above that of the pristine Li2NiO2. Furthermore, a graphite‖LiFePO4 pouch cell with a nominal capacity of 3000 mAh demonstrated a notably improved reversible capacity, energy density and cycle life through introducing 2 wt% Li2Ni0.7Cu0.3O2 additive, delivering a 6.2 mAh·g-1 higher initial discharge capacity and achieving around 5% improvement in capacity retentnion at 0.5P over 1000 cycles. Additionally, the post-mortem analyses testified that the Li2Ni0.7Cu0.3O2 additive could suppress solid electrolyte interphase (SEI) decomposition and homogenize the Li distribution, which benefits to stabilizing interface between graphite and electrolyte, and alleviating dendritic Li plating. In conclusion, the Li2Ni0.7Cu0.3O2 additive may offer advantages such as lower cost, lower delithiation voltage and higher prelithiation capacity compared with Li2NiO2, making it a promising candidate of cathode prelithiation additive for next-generation LIBs.

/
| 〈 |
|
〉 |