

碳纳米管穿插钴基卟啉金属有机框架催化ORR
收稿日期: 2024-05-24
修回日期: 2024-09-03
录用日期: 2024-09-06
网络出版日期: 2024-10-16
A CNT Intercalated Co Porphyrin-Based Metal Organic Framework Catalyst for Oxygen Reduction Reaction
#These authors contributed equally to this work.
Received date: 2024-05-24
Revised date: 2024-09-03
Accepted date: 2024-09-06
Online published: 2024-10-16
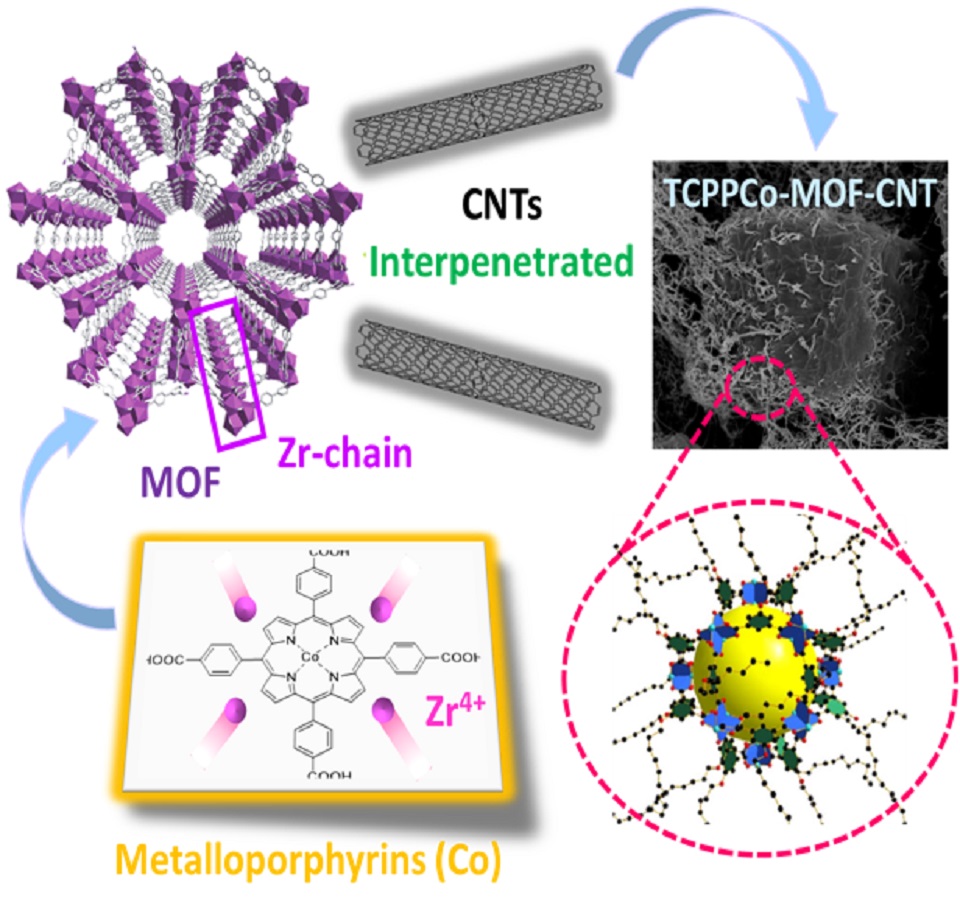
金属有机框架(MOF)材料作为典型的半导体材料,由于其本征的较差电子导电性,严重限制了其在燃料电池电催化领域的直接应用。为此,本文提出了一种解决策略,即在MOF晶体生长过程中加入碳纳米管(CNTs),最终合成了一种具有独特的CNT穿插在MOF晶体结构中的Co金属卟啉基的催化剂TCPPCo-MOF-CNT。物理表征表明,CNTs的插入不仅增强了催化剂整体的导电性,同时也保留了MOF本身的晶体结构和金属卟啉的原始特性。并且,由于CNTs的插入,在催化剂内部形成了大量促进传质的介孔结构,并产生了可以极大提升反应过程中传质效率的分级多孔结构。XPS揭示了插入的CNT中的碳原子改变了催化活性中心Co上的电子云密度,优化了催化中心的电子结构。在中性条件下,TCPPCo-MOF-CNT催化剂的半波电位达到0.77 V(vs. RHE),明显优于未插入CNT的单纯MOF材料制备的催化剂。最后,将TCPPCo-MOF-CNT作为阴极催化剂和Nafion-117组装成PEM微生物燃料电池(MFCs),MFCs显示其最大功率密度可达到约500 mW·m-2。因此,TCPPCo-MOF-CNT可作为一种有效的中性环境下ORR催化剂,同时此策略也为其他半导体材料提升电催化活性提供了一种方法。
何佩佩 , 师锦华 , 李笑语 , 刘明杰 , 方舟 , 和晶 , 李中坚 , 彭新生 , 和庆钢 . 碳纳米管穿插钴基卟啉金属有机框架催化ORR[J]. 电化学, 2025 , 31(1) : 2405241 . DOI: 10.61558/2993-074X.3502
The poor electronic conductivity of metal-organic framework (MOF) materials hinders their direct application in the field of electrocatalysis in fuel cells. Herein, we proposed a strategy of embedding carbon nanotubes (CNTs) during the growth process of MOF crystals, synthesizing a metalloporphyrin-based MOF catalyst TCPPCo-MOF-CNT with a unique CNT-intercalated MOF structure. Physical characterization revealed that the CNTs enhance the overall conductivity while retaining the original characteristics of the MOF and metalloporphyrin. Simultaneously, the insertion of CNTs generated adequate mesopores and created a hierarchical porous structure that enhances mass transfer efficiency. X-ray photoelectron spectroscopic analysis confirmed that the C atom in CNT changed the electron cloud density on the catalytic active center Co, optimizing the electronic structure. Consequently, the E1/2 of the TCPPCo-MOF-CNT catalyst under neutral conditions reached 0.77 V (vs. RHE), outperforming the catalyst without CNTs. When the TCPPCo-MOF-CNT was employed as the cathode catalyst in assembling microbial fuel cells (MFCs) with Nafion-117 as the proton exchange membrane, the maximum power density of MFCs reached approximately 500 mW·m-2.

| [1] | Nie Y, Li L, Wei Z D. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction[C]. Chem. Soc. Rev., 2015, 44(8): 2168-2201. |
| [2] | Chen Z W, Higgins D, Yu A P, Zhang L, Zhang J J. A review on non-precious metal electrocatalysts for PEM fuel cells[C]. Energy. Environ. Sci., 2011, 4(9): 3167-3192. |
| [3] | Hou J G, Wu Y Z, Zhang B, Cao S Y, Li Z W, Sun L C. Rational design of nanoarray architectures for electrocatalytic water splitting[J]. Adv. Funct. Mater., 2019, 29(20): 1808367. |
| [4] | Liang Z Z, Fan X, Lei H T, Qi J, Li Y Y, Gao J P, Huo M L, Yuan H T, Zhang W, Lin H P, Zheng H Q, Cao R. Cobalt-nitrogen-doped helical carbonaceous nanotubes as a class of efficient electrocatalysts for the oxygen reduction reaction[J]. Angew. Chem. Int. Ed., 2018, 57(40): 13187-13191. |
| [5] | Zhang C C, Yang H, Zhong D, Xu Y, Wang Y Z, Yuan Q, Liang Z Z, Wang B, Zhang W, Zheng H Q, Cheng T, Cao R. A yolk-shell structured metal-organic framework with encapsulated iron-porphyrin and its derived bimetallic nitrogen-doped porous carbon for an efficient oxygen reduction reaction[J]. J. Mater. Chem. A., 2020, 8(19): 9536-9544. |
| [6] | Wang M Q, Yang W H, Wang H H, Chen C, Zhou Z Y, Sun S G. Pyrolyzed Fe-N-C composite as an efficient non-precious metal catalyst for oxygen reduction reaction in acidic medium[J]. ACS. Catal., 2014, 4(11): 3928-3936. |
| [7] | Bezerra C W B, Zhang L, Lee K, Liu H, Marques A L B, Marques E P, Wang H, Zhang J. A review of Fe-N/C and Co-N/C catalysts for the oxygen reduction reaction[J]. Electrochim. Acta., 2008, 53(15): 4937-4951. |
| [8] | Fu K, Wang Y, Mao L C, Yang X X, Jin J H, Yang S L, Li G. Strongly coupled Co, N co-doped carbon nanotubes/graphene-like carbon nanosheets as efficient oxygen reduction electrocatalysts for primary zinc-air battery[J]. Chem. Eng. J., 2018, 351: 94-102. |
| [9] | Yin Y H, Zhang H B, Gao R Z, Wang A L, Mao X X, Dong H Y, Yang S T. In situ synthesis of metal embedded nitrogen doped carbon nanotubes as an electrocatalyst for the oxygen reduction reaction with high activity and stability[J]. RSC. Adv., 2018, 8(44): 25051-25056. |
| [10] | Zhang B B, Sun L C. Artificial photosynthesis: opportunities and challenges of molecular catalysts[J]. Chem. Soc. Rev., 2019, 48(7): 2216-2264. |
| [11] | Pegis M L, Wise C F, Martin D J, Mayer J M. Oxygen reduction by homogeneous molecular catalysts and electrocatalysts[J]. Chem. Rev., 2018, 118(5): 2340-2391. |
| [12] | Zhang W, Lai W Z, Cao R. Energy-related small molecule activation reactions: Oxygen reduction and hydrogen and oxygen evolution reactions catalyzed by porphyrin- and corrole-based systems[J]. Chem. Rev., 2017, 117(4): 3717-3797. |
| [13] | Guo X J, Wang N, Li X L, Zhang Z Y, Zhao J P, Ren W J, Ding S P, Xu G L, Li J F, Apfel U P, Zhang W, Cao R. Homolytic versus heterolytic hydrogen evolution reaction steered by a steric effect[J]. Angew. Chem. Int. Ed., 2020, 59(23): 8941-8946. |
| [14] | Bhunia S, Rana A, Roy P, Martin D J, Pegis M L, Roy B, Dey A. Rational design of mononuclear iron porphyrins for facile and selective 4e-/4H+ O2 reduction: Activation of O-O Bond by 2nd sphere hydrogen bonding[J]. J. Am. Chem. Soc., 2018, 140(30): 9444-9457. |
| [15] | Xie L S, Zhang X P, Zhao B, Li P, Qi J, Guo X N, Wang B, Lei H T, Zhang W, Apfel U P, Cao R. Enzyme-inspired iron porphyrins for improved electrocatalytic oxygen reduction and evolution reactions[J]. Angew. Chem. Int. Ed., 2021, 60(14): 7576-7581. |
| [16] | Costentin C, Saveant J M. Homogeneous molecular catalysis of electrochemical reactions: Manipulating intrinsic and operational factors for catalyst improvement[J]. J. Am. Chem. Soc., 2018, 140(48): 16669-16675. |
| [17] | Lv B, Li X L, Guo K, Ma J, Wang Y Z, Lei H T, Wang F, Jin X T, Zhang Q X, Zhang W, Long R, Xiong Y J, Apfel U P, Cao R. Controlling oxygen reduction selectivity through steric effects: Electrocatalytic two-electron and four-electron oxygen reduction with cobalt porphyrin atropisomers[J]. Angew. Chem. Int. Ed., 2021, 60(23): 12742-12746. |
| [18] | Brezny A C, Johnson S I, Raugei S, Mayer J M. Selectivity-determining steps in O2 reduction catalyzed by iron(tetramesitylporphyrin)[J]. J. Am. Chem. Soc., 2020, 142(9): 4108-4113. |
| [19] | Liu Y J, Zhou G J, Zhang Z Y, Lei H T, Yao Z, Li J F, Lin J, Cao R. significantly improved electrocatalytic oxygen reduction by an asymmetrical Pacman dinuclear cobalt(II) porphyrin-porphyrin dyad[J]. Chem. Sci., 2020, 11(1): 87-96. |
| [20] | Guo K, Lei H T, Li X L, Zhang Z Y, Wang Y B, Guo H B, Zhang W, Cao R. Alkali metal cation effects on electrocatalytic CO2 reduction with iron porphyrins[J]. Chin. J. Catal., 2021, 42(9): 1439-1444. |
| [21] | Sun L, Reddu V, Fisher A C, Wang X. Electrocatalytic reduction of carbon dioxide: opportunities with heterogeneous molecular catalysts[J]. Energ. Environ. Sci., 2020, 13(2): 374-403. |
| [22] | Corbin N, Zeng J, Williams K, Manthiram K. Heterogeneous molecular catalysts for electrocatalytic CO2 reduction[J]. Nano Res., 2019, 12(9): 2093-2125. |
| [23] | Sévery L, Szczerbinski J, Taskin M, Tuncay I, Nunes F B, Cignarella C, Tocci G, Blacque O, Osterwalder J, Zenobi R, Iannuzzi M, Tilley S D. Immobilization of molecular catalysts on electrode surfaces using host-guest interactions[J]. Nat. Chem., 2021, 13(6): 523-529. |
| [24] | Sun C F, Gobetto R, Nervi C. Recent advances in catalytic CO2 reduction by organometal complexes anchored on modified electrodes[J]. New. J. Chem., 2016, 40(7): 5656-5661. |
| [25] | Diercks C S, Liu Y Z, Cordova K E, Yaghi O M. The role of reticular chemistry in the design of CO2 reduction catalysts[J]. Nat. Mater., 2018, 17(10): 943-943. |
| [26] | Yang Z W, Chen J M, Qiu L Q, Xie W J, He L N. Molecular engineering of metal complexes for electrocatalytic carbon dioxide reduction: from adjustment of intrinsic activity to molecular immobilization[J]. Angew. Chem. Int. Edit., 2022, 61(44): e202205301 |
| [27] | Li X L, Lei H T, Xie L S, Wang N. Zhang W, Cao R. Metalloporphyrins as catalytic models for studying hydrogen and oxygen evolution and oxygen reduction reactions[J]. Acc. Chem. Res., 2022, 55(6): 878-892. |
| [28] | Xie W Y, Ling C, Huang Z Y, Chen W C, He S F, Si L P, Liu H Y. Metalloporphyrin doped rice husk-based biomass porous carbon materials as high performance electrocatalyst for oxygen reduction reaction in Zn-air battery[J]. Int. J. Hydrogen. Energ., 2024, 51: 857-868. |
| [29] | Leng F C, Liu H, Ding M L, Lin Q P, Jiang H L. Boosting photocatalytic hydrogen production of porphyrinic MOFs: The metal location in metalloporphyrin matters[J]. ACS. Catal., 2018, 8(5): 4583-4590. |
| [30] | Yang J, Wang Z, Li Y S, Zhuang Q X, Zhao W R, Gu J L. Porphyrinic MOFs for reversible fluorescent and colorimetric sensing of mercury(II) ions in aqueous phase[J]. Rsc. Adv., 2016, 6(74): 69807-69814. |
| [31] | Gil-San-Millan R, Koziel M, Bury W. Multivariate porphyrinic MOFs as precursors of nanoalloy catalysts for efficient dehydrogenation of hydrogen molecular carriers[J]. ACS. Appl. Energ. Mater., 2023, 6(18): 9136-9144. |
| [32] | Cichocka M O, Liang Z Z, Feng D W, Back S, Siahrostami S, Wang X, Samperisi L, Sun Y J, Xu H Y, Hedin N, Zheng H Q, Zou X D, Zhou H C, Huang Z H. A porphyrinic zirconium metal-organic framework for oxygen reduction reaction: Tailoring the spacing between active-sites through chain-based inorganic building units[J]. J. Am. Chem. Soc., 2020, 142(36): 15386-15395. |
| [33] | Guillerm V, Ragon F, Dan-Hardi M, Devic T, Vishnuvarthan M, Campo B, Vimont A, Clet G, Yang Q, Maurin G, Férey G, Vittadini A, Gross S, Serre C. A series of isoreticular, highly stable, porous zirconium oxide based metal-organic frameworks[J]. Angew. Chem. Int. Edit., 2012, 51(37): 9267-9271. |
/
| 〈 |
|
〉 |