

全固态钠离子电池:次世代电池竞赛中的领先竞争者
收稿日期: 2024-04-26
修回日期: 2024-05-26
录用日期: 2024-06-19
网络出版日期: 2024-06-19
All-Solid-State Sodium-Ion Batteries: A Leading Contender in the Next-Generation Battery Race
Received date: 2024-04-26
Revised date: 2024-05-26
Accepted date: 2024-06-19
Online published: 2024-06-19
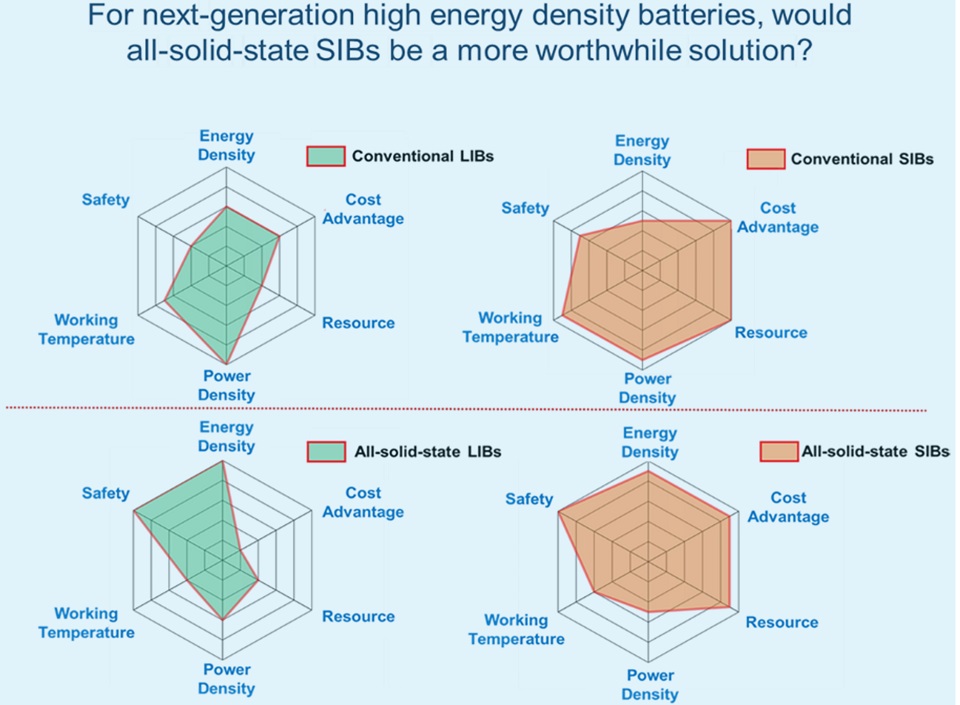
使用陶瓷电解质的全固态锂离子电池(LIBs)被认为是理想的可充电电池形式,因为它们具有高能量密度和安全性。然而,在追求全固态LIBs的过程中,锂资源层面的问题往往被选择性的忽视了。最具实用化潜力的富锂陶瓷电解质会使得全固态LIBs的锂消耗量是常规LIBs的数倍至数十倍。考虑到以当前的锂资源条件很难支撑全固态锂离子电池的可持续发展,另一种同样能够提供高能量密度和安全性双重优势的系统——全固态钠离子电池(SIBs),相比于锂离子电池具有更显著的可持续性优势,并有可能成为下一代高能量密度电池发展竞赛中的有力竞争者。然而,目前关于全固态钠离子电池的研究依然处于十分初步的阶段,本文简要介绍了全固态SIBs的研究现状,并通过对聚合物类材料,钠超离子导体(NASICON)类材料等固态钠离子导体的总结讨论,解释了全固态SIBs的可行性与潜在优势的来源。此外,本文还简要讨论了通过人工智能辅助开发固态钠离子导体的可行性,旨在激发研究人员的兴趣并吸引更多人关注到全固态SIBs这一领域中。
朱瑞杰 , 李泽辰 , 张伟 , 奈須滉 , 小林弘明 , 松井雅樹 . 全固态钠离子电池:次世代电池竞赛中的领先竞争者[J]. 电化学, 2024 , 30(12) : 2415002 . DOI: 10.61558/2993-074X.3476
All-solid-state lithium-ion batteries (LIBs) using ceramic electrolytes are considered the ideal form of rechargeable batteries due to their high energy density and safety. However, in the pursuit of all-solid-state LIBs, the issue of lithium resource availability is selectively overlooked. Considering that the amount of lithium required for all-solid-state LIBs is not sustainable with current lithium resources, another system that also offers the dual advantages of high energy density and safety— all-solid-state sodium-ion batteries (SIBs) —holds significant sustainable advantages and is likely to be the strong contender in the competition for developing next-generation high-energy-density batteries. This article briefly introduces the research status of all-solid-state SIBs, explains the sources of their advantages, and discusses potential approaches to the development of solid sodium-ion conductors, aiming to spark the interest of researchers and attract more attention to the field of all-solid-state SIBs.

| [1] | Blomgren G E. The development and future of lithium ion batteries[J]. J. Electrochem. Soc., 2016, 164(1): A5019-A5025. |
| [2] | Chen Y Q, Kang Y Q, Zhao Y, Wang L, Liu J L, Li Y X, Liang Z, He X M, Li X, Tavajohi N, Li B H. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards[J]. J. Energy Chem., 2021, 59: 83-99. |
| [3] | Quartarone E, Mustarelli P. Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives[J]. Chem. Soc. Rev., 2011, 40(5): 2525-2540. |
| [4] | Saccoccio M, Yu J, Lu Z, Kwok S C T, Wang J, Yeung K K, Yuen M M F, Ciucci F. Low temperature pulsed laser deposition of garnet Li6.4La3Zr1.4Ta0.6O12 films as all solid-state lithium battery electrolytes[J]. J. Power Sources, 2017, 365: 43-52. |
| [5] | Narayanan S, Ulissi U, Gibson J S, Chart Y A, Weatherup R S, Pasta M. Effect of current density on the solid electrolyte interphase formation at the lithium|Li6PS5Cl interface[J]. Nat. Commun., 2022, 13: 7237. |
| [6] | Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, Kamiyama T, Kato Y, Hama S, Kawamoto K, Mitsui A. A lithium superionic conductor[J]. Nat. Mater., 2011, 10(9): 682-686. |
| [7] | Miao X, Guan S D, Ma C, Li L L, Nan C W. Role of interfaces in solid-state batteries[J]. Adv. Mater., 2023, 35(50): 2206402. |
| [8] | Chen X Z, He W J, Ding L X, Wang S Q, Wang H H. Enhancing interfacial contact in all solid state batteries with a cathode-supported solid electrolyte membrane framework[J]. Energy Environ. Sci., 2019, 12(3): 938-944. |
| [9] | Kubota K, Komaba S. Review—practical issues and future perspective for Na-ion batteries[J]. J. Electrochem. Soc., 2015, 162(14): A2538-A2550. |
| [10] | Nayak P K, Yang L, Brehm W, Adelhelm P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises[J]. Angew. Chem. Int. Ed., 2018, 57(1): 102-120. |
| [11] | Oshima T, Kajita M, Okuno A. Development of sodium‐sulfur batteries[J]. Int. J. Appl. Ceram. Technol., 2005, 1(3): 269-276. |
| [12] | Vaalma C, Buchholz D, Weil M, Passerini S. A cost and resource analysis of sodium-ion batteries[J]. Nat. Rev. Mater., 2018, 3: 18013. |
| [13] | Tang W S, Yoshida K, Soloninin A V, Skoryunov R V, Babanova O A, Skripov A V, Dimitrievska M, Stavila V, Orimo S I, Udovic T J. Stabilizing superionic-conducting structures via mixed-anion solid solutions of monocarba-closo-borate salts[J]. ACS Energy Lett., 2016, 1(4): 659-664. |
| [14] | Serra Moreno J, Armand M, Berman M B, Greenbaum S G, Scrosati B, Panero S. Composite PEOn: NaTFSI polymer electrolyte: Preparation, thermal and electrochemical characterization[J]. J. Power Sources, 2014, 248: 695-702. |
| [15] | Goodenough J B, Hong H Y P, Kafalas J A. FAST Na+ - ion transport in skeleton structures[J]. Mater. Res. Bull., 1976, 11: 203-220. |
| [16] | Ruan Y L, Song S D, Liu J J, Liu P, Cheng B W, Song X Y, Battaglia V. Improved structural stability and ionic conductivity of Na3Zr2Si2PO12 solid electrolyte by rare earth metal substitutions[J]. Ceram. Int., 2017, 43(10): 7810-7815. |
| [17] | Zhang Z Z, Shi S Q, Hu Y S, Chen L Q. Sol-gel synthesis and conductivity properties of sodium ion solid state electrolytes Na3Zr2Si2PO12[J]. J. Inorg. Mater., 2013, 28(11): 1255-1260. |
| [18] | Shen L, Yang J, Liu G, Avdeev M, Yao X. High ionic conductivity and dendrite-resistant NASICON solid electrolyte for all-solid-state sodium batteries[J]. Mater. Today Energy, 2021, 20: 100691. |
| [19] | Ma Q L, Guin M, Naqash S, Tsai C L, Tietz F, Guillon O. Scandium-substituted Na3Zr2(SiO4)2(PO4) prepared by a solution-assisted solid-state reaction method as sodium-ion conductors[J]. Chem. Mat., 2016, 28(13): 4821-4828. |
| [20] | Yang J, Liu G Z, Avdeev M, Wan H L, Han F D, Shen L, Zou Z Y, Shi S Q, Hu Y S, Wang C S, Yao X Y. Ultrastable all-solid-state sodium rechargeable batteries[J], ACS Energy Lett., 2020, 5(9): 2835-2841. |
| [21] | Landesfeind J, Hosaka T, Graf M, Kubota K, Komaba S, Gasteiger H A. Comparison of ionic transport properties of non-aqueous lithium and sodium hexafluorophosphate electrolytes[J]. J. Electrochem. Soc., 2021, 168: 040538 |
| [22] | Chi X W, Zhang Y, Hao F, Kmiec S, Dong H, Xu R, Zhao K J, Ai Q, Terlier T, Wang L, Zhao L H, Guo L Q, Lou J, Xin H L, Martin S W, Yao Y. An electrochemically stable homogeneous glassy electrolyte formed at room temperature for all-solid-state sodium batteries[J]. Nat. Commun., 2022, 13(1): 2854. |
| [23] | Shao Y J, Zhong G M, Lu Y X, Liu L L, Zhao C L, Zhang Q Q, Hu Y S, Yang Y, Chen L Q. A novel NASICON-based glass-ceramic composite electrolyte with enhanced Na-ion conductivity[J]. Energy Storage Mater., 2019, 23: 514-521. |
| [24] | Fan S S, Lei M, Wu H, Hu J L, Yin C L, Liang T X, Li C L. A Na-rich fluorinated sulfate anti-perovskite with dual doping as solid electrolyte for Na metal solid state batteries[J]. Energy Storage Mater., 2020, 31: 87-94. |
| [25] | Hargreaves C J, Gaultois M W, Daniels L M, Watts E J, Kurlin V A, Moran M, Dang Y, Morris R, Morscher A, Thompson K, Wright M A, Prasa B E, Blanc F, Collins C M, Crawford C A, Duff B B, Evans J, Gamon J, Han G, Leube B T, Niu H, Perez A J, Robinson A, Rogan O, Sharp P M, Shoko E, Sonni M, Thomas W J, Vasylenko A, Wang L, Rosseinsky M J, Dyer M S. A database of experimentally measured lithium solid electrolyte conductivities evaluated with machine learning[J]. npj Comput. Mater., 2023, 9(1): 9. |
| [26] | Jo J, Choi E, Kim M, Min K. Machine learning-aided materials design platform for predicting the mechanical properties of Na-ion solid-state electrolytes[J]. ACS Appl. Energ. Mater., 2021, 4(8): 7862-7869. |
| [27] | Zhang Y, He X F, Chen Z Q, Bai Q, Nolan A M, Roberts C A, Banerjee D, Matsunaga T, Mo Y F, Ling C. Unsupervised discovery of solid-state lithium ion conductors[J]. Nat. Commun., 2019, 10(1): 5260. |
/
| 〈 |
|
〉 |