

纳米电极上单纳米气泡的伏安分析和电催化
收稿日期: 2024-04-09
修回日期: 2024-05-19
录用日期: 2024-05-22
网络出版日期: 2024-05-30
版权
Single Nanobubble Formation on Au Nanoelectrodes and Au@WS2 Nanoelectrodes: Voltammetric Analysis and Electrocatalysis
Received date: 2024-04-09
Revised date: 2024-05-19
Accepted date: 2024-05-22
Online published: 2024-05-30
Copyright
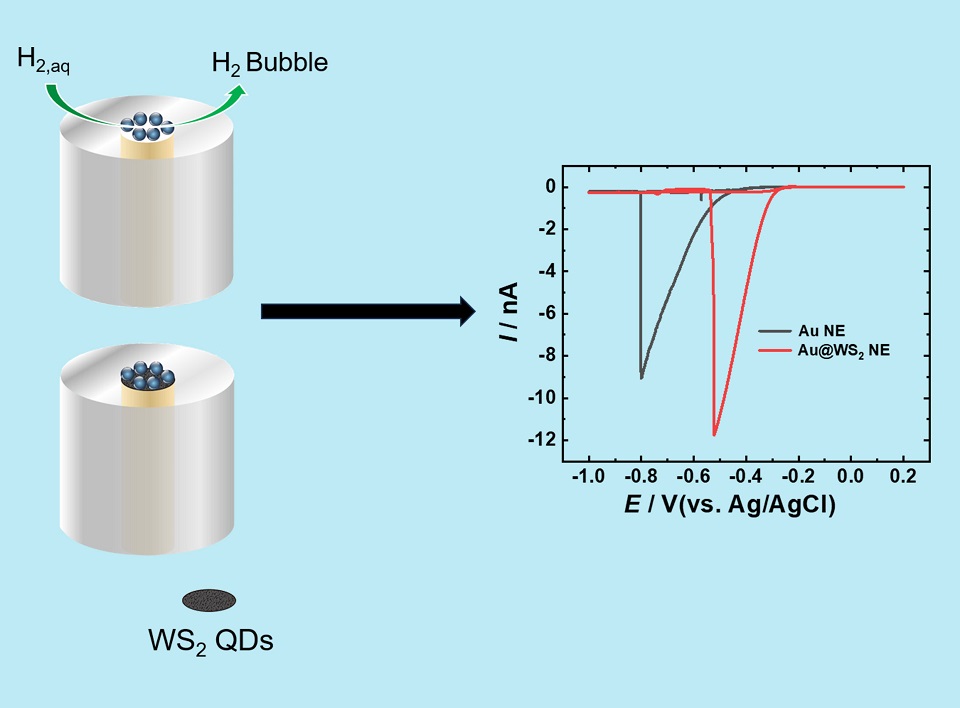
利用金纳米盘电极的极小尺寸(Au NEs,半径小于50 nm),研究了在纳米电极表面产生的单个氢纳米气泡,以评价其析氢性能。我们研究了Au NEs在不同浓度硫酸中的电化学行为,结果表明CV的形状随着硫酸浓度的增加从S型波逐渐变为峰型波。根据纳米气泡的形成机理,得出了产生单个纳米气泡的最小硫酸浓度,表明此时氢气在电极表面达到了临界过饱和,产生了单个纳米气泡和电化学峰型响应。并通过微动力学模型评价了金纳米电极和金@二硫化钨量子点纳米电极(Au@WS2 NEs)的析氢反应(HER)活性。结果表明,在Au NEs表面的临界溶解氢气浓度约为0.4 mol·L-1,相当于室温和大气压下溶于水中氢气过饱和度的500倍。此外,通过对单个纳米气泡形成前的电流强度的微动力学分析,发现Au@WS2 NEs和Au NEs析氢反应的决速步骤分别为Heyrovsky step和Volmer step,Au@WS2 NEs决速步的标准速率常数(k0)约为Au NEs的12倍,表明Au@WS2 NEs具有更高的HER活性。随着HER活性的增加,气泡形成电位转向更正的电位。这项工作利用极小尺寸的纳米电极甚至包括分子尺寸的纳米电极对其表面产生的单个氢纳米气泡进行研究,为纳米气泡电化学研究提供了基础,并为后续基于气泡的应用提供了新的思路,可以帮助我们设计和筛选应用于基础电化学、电催化和能源相关领域的新型纳米材料,特别是在单个实体水平上。
罗贤准 , 陈晓虎 , 李永新 . 纳米电极上单纳米气泡的伏安分析和电催化[J]. 电化学, 2024 , 30(10) : 2414001 . DOI: 10.61558/2993-074X.3475
Taking advantage of the extremely small size of the gold nanodisk electrode, the single hydrogen nanobubble generated on the surface of the nanoelectrode was studied to evaluate its hydrogen evolution performance. It was found that compared with the bare gold nanodisk electrode, the bubble formation potential of the gold nanodisk electrode modified with tungsten disulfide quantum dots (WS2 QDs) on the surface was more positive, indicating that its hydrogen evolution activity was higher. Microdynamic model analysis shows that the average standard rate constant of the rate-determining step of the hydrogen evolution reaction of gold nanoelectrodes modified with WS2 QDs is approximately 12 times larger than that of gold nanoelectrodes. This work based on the formation of nanobubbles provides new ideas for the design and performance evaluation of hydrogen evolution reaction catalysts.

Key words: Nanoelectrode; Nanobubble; Electrocatalysis
| [1] | Khaselev O, Turner J A. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting[J]. Science, 1998, 280(5362): 425-427. |
| [2] | Wang X, Maeda K, Thomas A, Takanabe K, Xin G, Carlsson J M, Domen K, Antonietti M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nat. Mater., 2009, 8(1): 76-80. |
| [3] | Liang Y Y, Li Y G, Wang H L, Zhou J G, Wang J, Regier T, Dai H J. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction[J]. Nat. Mater., 2011, 10(10): 780-786. |
| [4] | Cheng Y, Xu C, Jia L, Gale J D, Zhang L, Liu C, Shen P K. Pristine carbon nanotubes as non-metal electrocatalysts for oxygen evolution reaction of water splitting[J]. Appl. Catal. B-Environ., 2015, 163: 96-104. |
| [5] | Rees N V, Compton R G. Carbon-free energy: A review of ammonia-and hydrazine-based electrochemical fuel cells[J]. Energy Environ. Sci., 2011, 4(4): 1255-1260. |
| [6] | Meng Y Y, Zou X X, Huang X X, Goswami A, Liu Z W, Asefa T. Polypyrrole-derived nitrogen and oxygen co-doped mesoporous carbons as efficient metal-free electrocatalyst for hydrazine oxidation[J]. Adv. Mater., 2014, 26(37): 6510-6516. |
| [7] | Van Der Linde P, Pe?as-López P, Soto á M, Van Der Meer D, Lohse D, Gardeniers H, Rivas D F. Gas bubble evolution on microstructured silicon substrates[J]. Energy Environ. Sci., 2018, 11(12): 3452-3462. |
| [8] | Zhao X, Ren H, Luo L. Gas bubbles in electrochemical gas evolution reactions[J]. Langmuir, 2019, 35(16): 5392-5408. |
| [9] | Angulo A, van der Linde P, Gardeniers H, Modestino M, Rivas D F. Influence of bubbles on the energy conversion efficiency of electrochemical reactors[J]. Joule, 2020, 4(3): 555-579. |
| [10] | Graziano G. Forever blowing nanobubbles[J]. Nat. Rev. Chem., 2020, 4(10): 506-506. |
| [11] | Liu Y L, Jin C, Liu Y W, Chen Q J. Recent progress in gas nanobubble electrochemistry[J]. Sci. China Chem., 2021, 51(3): 310-322. |
| [12] | Luo L, White H S. Electrogeneration of single nanobubbles at sub-50-nm-radius platinum nanodisk electrodes[J]. Langmuir, 2013, 29(35): 11169-11175. |
| [13] | Chen Q, Luo L, Faraji H, Feldberg S W, White H S. Electrochemical measurements of single H2 nanobubble nucleation and stability at Pt nanoelectrodes[J]. J. Phys. Chem. Lett., 2014, 5(20): 3539-3544. |
| [14] | Chen Q, Luo L, White H S. Electrochemical generation of a hydrogen bubble at a recessed platinum nanopore electrode[J]. Langmuir, 2015, 31(15): 4573-4581. |
| [15] | Chen Q, Wiedenroth H S, German S R, White H S. Electrochemical nucleation of stable N2 nanobubbles at Pt nanoelectrodes[J]. J. Am. Chem. Soc., 2015, 137(37): 12064-12069. |
| [16] | German S R, Edwards M A, Chen Q, Liu Y, Luo L, White H S. Electrochemistry of single nanobubbles. Estimating the critical size of bubble-forming nuclei for gas-evolving electrode reactions[J]. Faraday Discuss., 2016, 193: 223-240. |
| [17] | Ren H, German S R, Edwards M A, Chen Q, White H S. Electrochemical generation of individual O2 nanobubbles via H2O2 oxidation[J]. J. Phys. Chem. Lett., 2017, 8(11): 2450-2454. |
| [18] | Ren H, Edwards M A, Wang Y, White H S. Electrochemically controlled nucleation of single CO2 nanobubbles via formate oxidation at Pt nanoelectrodes[J]. J. Phys. Chem. Lett., 2020, 11(4): 1291-1296. |
| [19] | Qiu X, Wei H F, Li R J, Li Y X. Electrochemical and electrocatalytic performance of single Au@Pt/Au bimetallic nanoparticles[J]. J. Alloy. Compd., 2023, 956: 170365. |
| [20] | Chen W, Wang H, Tang H R, Yang C, Li Y X. Unique voltammetry of silver nanoparticles: From single particle to aggregates[J]. Anal. Chem., 2019, 91(22): 14188-14191. |
| [21] | Duan X H, Li N, Wang G N, Su X G. High sensitive ratiometric fluorescence analysis of trypsin and dithiothreitol based on WS2 QDs[J]. Talanta, 2020, 219: 121171. |
| [22] | Guo X R, Wang Y, Wu F Y, Ni Y N, Kokot S. The use of tungsten disulfide dots as highly selective, fluorescent probes for analysis of nitrofurazone[J]. Talanta, 2015, 144: 1036-1043. |
| [23] | Pakiari A, Jamshidi Z. Nature and strength of M- S Bonds (M= Au, Ag, and Cu) in binary alloy gold clusters[J]. J. Phys. Chem. A, 2010, 114(34): 9212-9221. |
| [24] | Hua H M, Liu Y, Wang D M, Li Y X. Size-dependent voltammetry at single silver nanoelectrodes[J]. Anal. Chem., 2018, 90(16): 9677-9681. |
| [25] | Li Y X, Wu Q Q, Jiao S F, Xu C D, Wang L. Single Pt nanowire electrode: preparation, electrochemistry, and electrocatalysis[J]. Anal. Chem., 2013, 85(8): 4135-4140. |
| [26] | Watkins J J, Chen J, White H S, Abruna H D, Maisonhaute E, Amatore C. Zeptomole voltammetric detection and electron-transfer rate measurements using platinum electrodes of nanometer dimensions[J]. Anal. Chem., 2003, 75(16): 3962-3971. |
| [27] | Cheng Z L, Ma L, Liu Z. Hydrothermal-assisted grinding route for WS2 quantum dots (QDs) from nanosheets with preferable tribological performance[J]. Chin. Chem. Lett., 2021, 32(1): 583-586. |
| [28] | Yan Z L, Fu L J, Yang H M, Ouyang J. Amino-functionalized hierarchical porous SiO2-AlOOH composite nanosheets with enhanced adsorption performance[J]. J. Hazard. Mater., 2018, 344: 1090-1100. |
| [29] | Bayat A, Saievar-Iranizad E. Synthesis of blue photoluminescent WS2 quantum dots via ultrasonic cavitation[J]. J. Lumines., 2017, 185: 236-240. |
| [30] | Yan Y H, Zhang C L, Gu W, Ding C P, Li X C, Xian Y Z. Facile synthesis of water-soluble WS2quantum dots for turn-on fluorescent measurement of lipoic acid[J]. J. Phys. Chem. C, 2016, 120(22): 12170-12177. |
| [31] | Lin L X, Xu Y X, Zhang S W, Ross I M, Ong A C M, Allwood D A. Fabrication of luminescent monolayered tungsten dichalcogenides quantum dots with giant spin-valley coupling[J]. ACS nano, 2013, 7(9): 8214-8223. |
| [32] | Xu S S, Gao X M, Hu M, Sun J Y, Wang D S, Zhou F, Weng L J, Liu W M. Morphology evolution of Ag alloyed WS2 films and the significantly enhanced mechanical and tribological properties[J]. Surf. Coat. Technol., 2014, 238: 197-206. |
| [33] | Wang Y, Liu Y, Zhang J F, Wu J J, Xu H, Wen X W, Zhang X, Tiwary C S, Yang W, Vajtai R, Zhang Y, Chopra N, Odeh I N, Wu Y C, Ajayan P M. Cryo-mediated exfoliation and fracturing of layered materials into 2D quantum dots[J]. Sci. Adv., 2017, 3(12): e1701500. |
| [34] | Shi F Y, Du J R, Han Q, Zhang F R, Wang K, Kan Z T, Wang L, Li C Y, Xu L. Integrated wearable foam modified with WS2nanosheets@MoS2 quantum dots for oral disease diagnosis and healthcare monitoring[J]. Chem. Eng. J., 2023, 477: 146800. |
| [35] | Edwards M A, White H S, Ren H. Voltammetric determination of the stochastic formation rate and geometry of individual H2, N2, and O2 bubble nuclei[J]. ACS Nano, 2019, 13(6): 6330-6340. |
| [36] | Chen Q, Ranaweera R, Luo L. Hydrogen bubble formation at hydrogen-insertion electrodes[J]. J. Phys. Chem. C, 2018, 122(27): 15421-15426. |
| [37] | Chen Q J, Luo L. Correlation between gas bubble formation and hydrogen evolution reaction kinetics at nanoelectrodes[J]. Langmuir, 2018, 34(15): 4554-4559. |
| [38] | Wei H F, Wang H, Tang H R, Li Y X. Voltammetric analysis of single nanobubble formation on Ag and Ag@MoS2 nanoelectrodes[J]. J. Phys. Chem. C, 2021, 125(5): 3073-3080. |
| [39] | Sheng W, Gasteiger H A, Shao-Horn Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs alkaline electrolytes[J]. J. Electrochem. Soc., 2010, 157(11): B1529. |
| [40] | Durst J, Siebel A, Simon C, Hasché F, Herranz J, Gasteiger H A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism[J]. Energy Environ. Sci., 2014, 7(7): 2255-2260. |
| [41] | Shinagawa T, Garcia-Esparza A T, Takanabe K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion[J]. Sci Rep, 2015, 5(1): 13801. |
| [42] | Hill C M, Kim J, Bard A J. Electrochemistry at a metal nanoparticle on a Tunneling film: A steady-state model of current densities at a tunneling ultramicroelectrode[J]. J. Am. Chem. Soc., 2015, 137(35): 11321-11326. |
| [43] | Defnet P A, Han C, Zhang B. Temporally-resolved ultrafast hydrogen adsorption and evolution on single platinum nanoparticles[J]. Anal. Chem., 2019, 91(6): 4023-4030. |
| [44] | Mariano R G, McKelvey K, White H S, Kanan M W. Selective increase in CO2 electroreduction activity at grain-boundary surface terminations[J]. Science, 2017, 358(6367): 1187-1192. |
| [45] | Liu C M, Lin H W, Huang Y S, Chu Y C, Chen C, Lyu D R, Chen K N, Tu K N. Low-temperature direct copper-to-copper bonding enabled by creep on (111) surfaces of nanotwinned Cu[J]. Sci Rep, 2015, 5(1): 9734. |
| [46] | Aaronson B D B, Chen C H, Li H, Koper M T M, Lai S C S, Unwin P R. Pseudo-single-crystal electrochemistry on polycrystalline electrodes: Visualizing activity at grains and grain boundaries on platinum for the Fe2+/Fe3+ redox reaction[J]. J. Am. Chem. Soc., 2013, 135(10): 3873-3880. |
| [47] | Chen C H, Meadows K E, Cuharuc A, Lai S C S, Unwin P R. High resolution mapping of oxygen reduction reaction kinetics at polycrystalline platinum electrodes[J]. Phys. Chem. Chem. Phys., 2014, 16(34): 18545-18552. |
/
| 〈 |
|
〉 |