

钯催化电化学烯丙位4-吡啶化反应中的配体作用研究
收稿日期: 2023-08-14
修回日期: 2023-09-04
录用日期: 2023-12-08
网络出版日期: 2023-12-24
Comparison of Ligands in Palladium-Catalyzed Electrochemical Allyl 4-Pyridinylation
Received date: 2023-08-14
Revised date: 2023-09-04
Accepted date: 2023-12-08
Online published: 2023-12-24
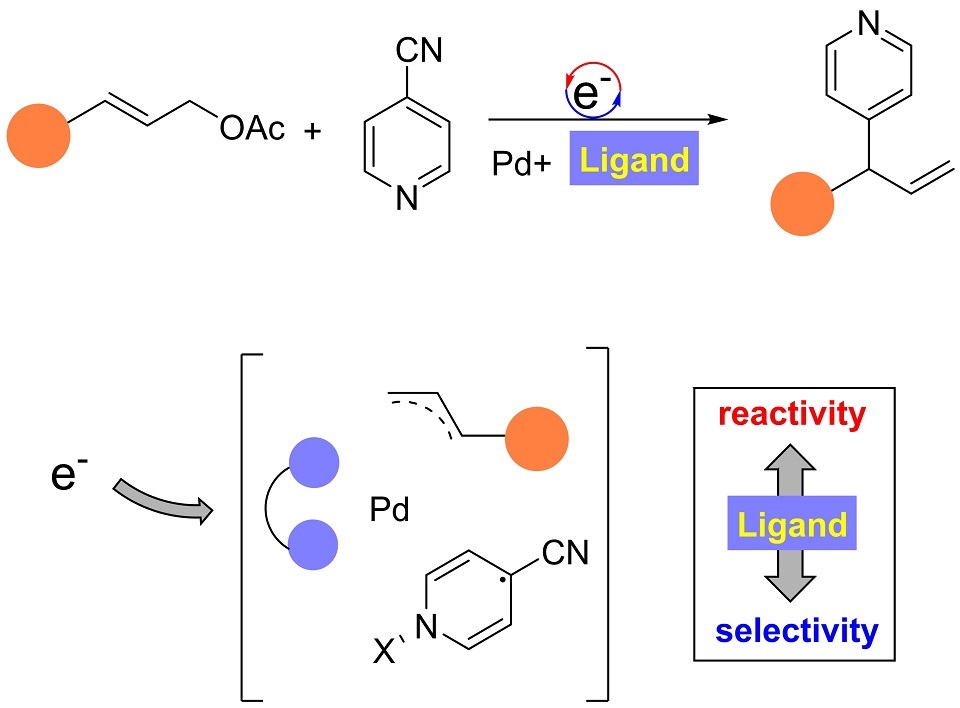
过渡金属络合物在电化学合成中获得了广泛的应用,其中配体对于络合物在电场中稳定性、催化活性以及选择性的影响还了解有限。4-氰基-吡啶作为一种高效吡啶化试剂,在自由基化学中获得广泛应用。在前期的工作中,我们实现了电化学条件下,手性双膦配体钯络合物催化4-氰基-吡啶与烯丙基醋酸酯反应,构建了多种手性烯丙基吡啶化合物。我们发现双膦配体对反应有着关键的作用,决定着反应的活性、区域选择性和对映选择性。在本工作中,我们系统性地研究了多种双膦配体钯金属络合物,在烯丙基醋酸酯与氰基吡啶的电化学还原偶联过程中的性质。通过控制实验,电化学分析以及理论计算等方法,我们揭示了双膦配体对于络合物稳定性及反应区域选择性的影响。进而,我们发现在电场条件下存在一个非稳定价态的过渡金属络合物。这个非稳定价态的过渡金属络合物中,双膦配体可以将电荷和自旋密度分散于整个络合物之中,而不是局限于金属离子之上。这样,络合物既可以作为电子转移催化剂,也可以作为过渡金属催化剂,同时控制整个电子转移过程以及成键过程。我们认为这种配体与金属在电场条件下的非稳定价态络合物,展现了电化学条件下过渡金属催化的独特能力,这有助于发展未来的新型的电化学催化体系。同时,我们还发现锌电极至关重要,其不仅可以活化4-氰基吡啶,还可以淬灭氰根离子,展现出Lewis酸性金属离子的特殊用途。
丁伟杰 , 杨春晖 , 冯钟涛 , 陆仕荣 , 程旭 . 钯催化电化学烯丙位4-吡啶化反应中的配体作用研究[J]. 电化学, 2024 , 30(5) : 2313003 . DOI: 10.61558/2993-074X.3438
4-CN-pyridine is a widely applied 4-pyridinylation reagent for diverse transformations. Conventionally, the reaction proceeds via an open-shell radical cross-coupling pathway. Following our previous study, in this work, we report the Pd-catalyzed allyl 4-pyrinylation reaction under electrochemical conditions. The reaction proceeds via radical-polar crossover pathway in which the role of phosphine ligand in reactivity and selectivity was extensively investigated.

Key words: Electrochemistry; Palladium; Phosphine; Pyridinylation; Allyl
| [1] | Ataf A A, Adnan S, Zarif G, Nasir R, Amin B, Bhajan L, Ezzat K. A review on the medicinal importance of Pyridine Derivatives[J]. J. Drug Des. Med. Chem., 2015, 1(1): 1-11. |
| [2] | Nakao Y, Yada A, Satoh J, Ebata S, Oda S, Hiyama T. Arylcyanation of norbornene and norbornadiene catalyzed by nickel[J]. Chem. Lett., 2006, 35(7): 790-791. |
| [3] | McNally A, Prier C K, MacMillan D W C. Discovery of an α-amino C-H arylation reaction using the strategy of accelerated serendipity[J]. Science, 2011, 334(6059): 1114-1117. |
| [4] | Pirnot M T, Rankic D A, Martin D B C, MacMillan D W C. Photoredox activation for the direct β-arylation of ketones and aldehydes[J]. Science, 2013, 339(6127): 1593-1596. |
| [5] | Qvortrup K, Rankic D A, MacMillan D W C. A general strategy for organocatalytic activation of C-H bonds via photoredox catalysis: Direct arylation of benzylic ethers[J]. J. Am. Chem. Soc., 2014, 136(2): 626-629. |
| [6] | Cuthbertson J D, MacMillan D W C. The direct arylation of allylic sp3 C-H bonds via organic and photoredox catalysis[J]. Nature, 2015, 519(7541): 74-77. |
| [7] | Lipp B, Lipp A, Detert H, Opatz T. Light-induced alkylation of (hetero)aromatic nitriles in a transition-metal-free C-C-bond metathesis[J]. Org. Lett., 2017, 19(8): 2054-2057. |
| [8] | Lima F, Kabeshov M A, Tran D N, Battilocchio C, Sedelmeier J, Sedelmeier G, Schenkel B, Ley S V. Visible light activation of boronic esters enables efficient photoredox C(sp2)-C(sp3) cross-couplings in flow[J]. Angew. Chem. Int. Ed., 2016, 55(45): 14085-14089. |
| [9] | Lipp B, Nauth A M, Opatz T. Transition-metal-free decarboxylative photoredox coupling of carboxylic acids and alcohols with aromatic nitriles[J]. Org. Chem., 2016, 81(15): 6875-6882. |
| [10] | Gao L Z, Wang G Q, Cao J, Chen H, Gu Y M, Liu X T, Cheng X, Ma J, Li S H. Lewis acid-catalyzed selective reductive decarboxylative pyridylation of N-hydroxyphthalimide esters: Synthesis of congested pyridine-substituted quaternary carbons[J]. ACS Catal., 2019, 9(11): 10142-10151. |
| [11] | Shi J L, Yuan T, Zheng M F, Wang X C. Metal-free heterogeneous semiconductor for visible-light photocatalytic decarboxylation of carboxylic acids[J]. ACS Catal., 2021, 11(5): 3040-3047. |
| [12] | Wang G Q, Cao J, Gao L Z, Chen W X, Huang W H, Cheng X, Li S H. Metal-free synthesis of C-4 substituted pyridine derivatives using pyridine-boryl radicals via a radical addition/coupling mechanism: A combined computational and experimental study[J]. J. Am. Chem. Soc., 2017, 139(10): 3904-3910. |
| [13] | Zhang X, Yang C, Gao H, Wang L, Guo L, Xia W J. Reductive arylation of aliphatic and aromatic aldehydes with cyanoarenes by electrolysis for the synthesis of alcohols[J]. Org. Lett., 2021, 23(9): 3472-3476. |
| [14] | Ding W J, Sheng J, Li J, Cheng X. Electroreductive 4-pyridylation of unsaturated compounds using gaseous ammonia as a hydrogen source[J]. Org. Chem. Front, 2022, 9(10): 2634-2639. |
| [15] | Cao J, Wang G Q, Gao L Z, Chen H, Liu X T, Cheng X, Li S H. Perfluoroalkylative pyridylation of alkenes via 4-cyanopyridine-boryl radicals[J]. Chem. Sci., 2019, 10(9): 2767-2772. |
| [16] | Chen J, Zhu S Q, Qin J, Chu L L. Intermolecular, redox-neutral azidoarylation of alkenes via photoredox catalysis[J]. Chem. Commun., 2019, 55(16): 2336-2339. |
| [17] | Lipp B, Kammer L M, Kücükdisli M, Luque A, Kühlborn J, Pusch S, Matuleviciute G, Schollmeyer D, Sackus A, Opatz T. Visible light-induced sulfonylation/arylation of styrenes in a double radical three-component photoredox reaction[J]. Chem. Eur. J., 2019, 25(38): 8965-8969. |
| [18] | Zhu S Q, Qin J, Wang F, Li H, Chu L L. Photoredox-catalyzed branch-selective pyridylation of alkenes for the expedient synthesis of Triprolidine[J]. Nat. Commun., 2019, 10: 749. |
| [19] | Betori R C, Scheidt K A. Reductive arylation of arylidene malonates using photoredox catalysis (Retracted Article)[J]. ACS Catal., 2019, 9(11): 10350-10357. |
| [20] | Qi J, Zhang F L, Jin J K, Zhao Q, Li B, Liu L X, Wang Y F. New radical borylation pathways for organoboron synthesis enabled by photoredox catalysis[J]. Angew. Chem. Int. Ed., 2020, 59(31): 12876-12884. |
| [21] | Zhang S, Li L J, Li X R, Zhang J Q, Xu K, Li G G, Findlater M. Electroreductive 4-pyridylation of electron-deficient alkenes with assistance of ni(acac)2[J]. Org. Lett., 2020, 22(9): 3570-3575. |
| [22] | Li Y J, Han C J, Wang Y Y, Huang X, Zhao X W, Qiao B K, Jiang Z Y. Catalytic asymmetric reductive azaarylation of olefins via enantioselective radical coupling[J]. J. Am. Chem. Soc., 2022, 144(17): 7805-7814. |
| [23] | Miao M, Liao L L, Cao G M, Zhou W J, Yu D G. Visible-light-mediated external-reductant-free reductive cross coupling of benzylammonium salts with (hetero)aryl nitriles[J]. Sci. Chin. Chem., 2019, 62(11): 1519-1524. |
| [24] | Lehnherr D, Lam Y H, Nicastri M C, Liu J C, Newman J A, Regalado E L, DiRocco D A, Rovis T. Electrochemical synthesis of hindered primary and secondary amines via proton-coupled electron transfer[J]. J. Am. Chem. Soc., 2020, 142(1): 468-478. |
| [25] | Wen J W, Yang X T, Yan K L, Qin H Y, Ma J, Sun X J, Yang J J, Wang H. Electroreductive C3 pyridylation of quinoxalin-2(1H)-ones: An effective way to access bidentate nitrogen ligands[J]. Org. Lett., 2021, 23(3): 1081-1085. |
| [26] | Jahn U. Radicals in transition metal catalyzed reactions? Transition metal catalyzed radical reactions? A fruitful interplay anyway[J]. Top. Curr. Chem., 2011, 320, 323-451. |
| [27] | Twilton J, Le C, Zhang P, Shaw M H, Evans R W, MacMillan D W C. The merger of transition metal and photocatalysis[J]. Nat. Rev. Chem., 2017, 1(7): 0052. |
| [28] | Lu J Q, Wang Y K, McCallum T, Fu N K. Harnessing radical chemistry via electrochemical transition metal catalysis[J]. iScience, 2020, 23(12): 101796. |
| [29] | Cheng X, Lei A W, Mei T S, Xu H C, Xu K, Zeng C C. Recent applications of homogeneous catalysis in electrochemical organic synthesis[J]. CCS Chem., 2022, 4: 1120-1152. |
| [30] | Ma C, Fang P, Liu Z R, Xu S S, Xu K, Cheng X, Lei A W, Xu H C, Zeng C C, Mei T S. Recent advances in organic electrosynthesis employing transition metal complexes as electrocatalysts[J]. Sci. Bull., 2021, 66(23): 2412-2429. |
| [31] | Zhang W, Wang F, McCann S D, Wang D H, Chen P H, Stahl S S, Liu G S. Enantioselective cyanation of benzylic C-H bonds via copper-catalyzed radical relay[J]. Science, 2016, 353(6303): 1014-1018. |
| [32] | Ge L, Zhou H, Chiou M F, Jiang H M, Jian W J, Ye C Q, Li X Y, Zhu X T, Xiong H G, Li Y J, Song L J, Zhang X H, Bao H L. Iron-catalysed asymmetric carboazidation of styrenes[J]. Nat. Catal., 2021, 4(1): 28-35. |
| [33] | Zhang C, Li Z L, Gu Q S, Liu X Y. Catalytic enantioselective C(sp3)-H functionalization involving radical intermediates[J]. Nat. Commun., 2021, 12(1): 475. |
| [34] | Zhou Q, Chin M, Fu Y, Liu P, Yang Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450[J]. Science, 2021, 374(6575): 1612-1616. |
| [35] | Ding W J, Li M F, Fan J K, Cheng X. Palladium-catalyzed asymmetric allylic 4-pyridinylation via electroreductive substitution reaction[J]. Nat. Commun., 2022, 13(1): 5642-5652. |
| [36] | Pitzer L, Schwarz J L, Glorius F. Reductive radical-polar crossover: Traditional electrophiles in modern radical reactions[J]. Chem. Sci., 2019, 10(36): 8285-8291. |
| [37] | Wiles R J, Molander G A. Photoredox-mediated net-neutral radical/polar crossover reactions[J]. Isr. J. Chem., 2020, 60(3-4): 281-293. |
| [38] | Sharma S, Singh J, Sharma A. Visible light assisted radical-polar/polar-radical crossover reactions in organic synthesis[J]. Adv. Synth. Catal., 2021, 363(13): 3146-3169. |
| [39] | Jiao K J, Li Z M, Xu X T, Zhang L P, Li Y Q, Zhang K, Mei T S. Palladium-catalyzed reductive electrocarboxylation of allyl esters with carbon dioxide[J]. Org. Chem. Front., 2018, 5(14): 2244-2248. |
| [40] | Zhang H H, Zhao J J, Yu S Y. Enantioselective allylic alkylation with 4-alkyl-1,4-dihydropyridines enabled by photoredox/palladium cocatalysis[J]. J. Am. Chem. Soc., 2018, 140(49): 16914-16919. |
| [41] | Zhang H H, Zhao J J, Yu S Y. Enantioselective α-allylation of anilines enabled by a combined palladium and photoredox catalytic system[J]. ACS Catal., 2020, 10(8): 4710-4716. |
| [42] | Zhang H H, Tang M H, Zhao J J, Song C H, Yu S Y. Enantioselective reductive homocoupling of allylic acetates enabled by dual photoredox/palladium catalysis: Access to C2-symmetrical 1,5-dienes[J]. J. Am. Chem. Soc., 2021, 143(32): 12836-12846. |
/
| 〈 |
|
〉 |