

电化学促进的镍催化的α-氰基乙酸酯的α-芳基化反应
收稿日期: 2023-08-27
修回日期: 2023-11-12
录用日期: 2023-11-14
网络出版日期: 2023-11-21
Nickel-Catalyzed α-Arylation of α-Cyanoacetates Enabled by Electrochemistry
Received date: 2023-08-27
Revised date: 2023-11-12
Accepted date: 2023-11-14
Online published: 2023-11-21
李子萌 , 李章健 , 方萍 , 梅天胜 . 电化学促进的镍催化的α-氰基乙酸酯的α-芳基化反应[J]. 电化学, 2024 , 30(5) : 2313004 . DOI: 10.61558/2993-074X.3435
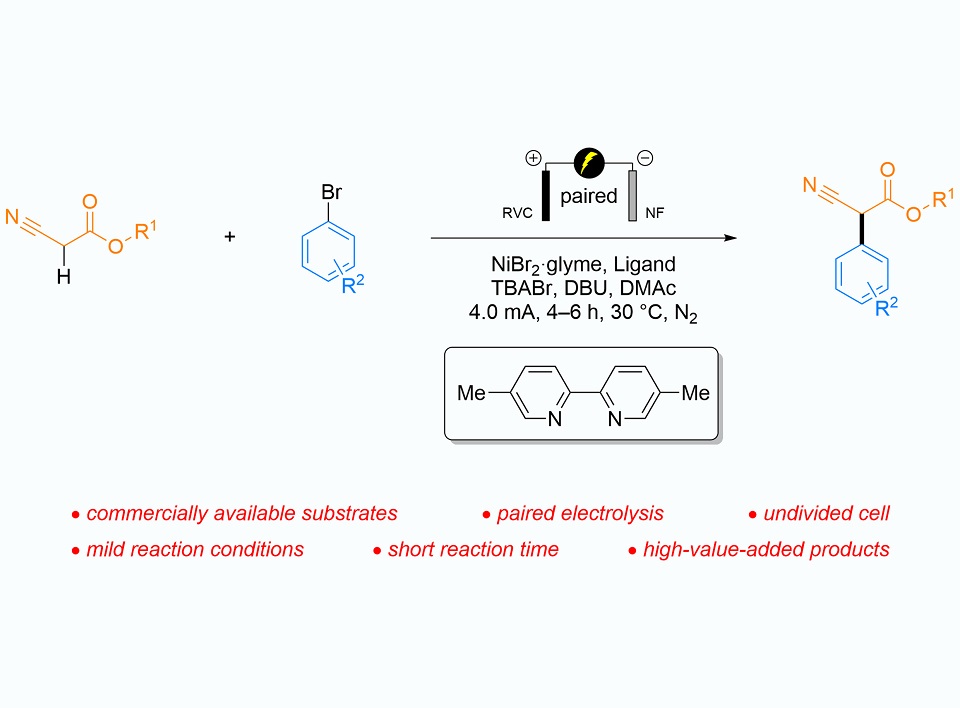
β-Amino acids have a wide range of applications in the field of pharmaceuticals. Utilizing a combination strategy of nickel catalysis and paired electrolysis, a catalytic α-arylation protocol of carbonyl compounds has been developed. This protocol affords various α-aryl-α-cyanoacetates, which can be reduced to high-value-added α-aryl-β-amino acids. The cross-coupling reaction of electron-deficient aryl bromides with α-cyanoacetates achieves the expected products with good yields and functional group compatibility under mild conditions. Excessive electron-richness in initial aryl bromides facilitates the self-coupling of desired products. DFT calculations confirm that the presence of electron-rich aryl substitutions decreases the reduction potentials of the product anions, making them more susceptible to oxidation at the anode. Based on electroanalyses and mechanistic studies, it is proposed that the enolate intermediate, rather than the radical intermediate, participates in the catalytic cycle.
| [1] | Lelais G, Seebach D. β2-amino acids—syntheses, occurrence in natural products, and components of β-peptides[J]. Pept. Sci., 2004, 76(3): 206-243. |
| [2] | Seebach D, Overhand M, Kühnle F N M, Martinoni B, Oberer L, Hommel U, Widmer H. β-peptides: Synthesis by Arndt-Eistert homologation with concomitant peptide coupling. Structure determination by NMR and CD spectroscopy and by X-ray crystallography. Helical secondary structure of a β-hexapeptide in solution and its stability towards pepsin[J]. Helv. Chim. Acta, 1996, 79(4): 913-941. |
| [3] | Appella D H, Christianson L A, Karle I L, Powell D R, Gellman S H. β-peptide foldamers: robust helix formation in a new family of β-amino acid oligomers[J]. J. Am. Chem. Soc., 1996, 118(51): 13071-13072. |
| [4] | Seebach D, Abele S, Schreiber J V, Martinoni B, Nussbaum A K, Schild H, Schulz H, Hennecke H, Woessner R, Bitsch F. Biological and pharmacokinetic studies with β-peptides[J]. Chimia, 1998, 52(12): 734-739. |
| [5] | Podlech J, Seebach D. The arndt-eistert reaction in peptide chemistry: A facile access to homopeptides[J]. Angew. Chem. Int. Ed., 1995, 34(4): 471-472. |
| [6] | Semmelhack M F, Stauffer R D, Rogerson T D. Nucleophilic aromatic substitution via a new nickel-catalyzed process and via the SRN1 reaction. Improved synthesis of cephalotaxinone[J]. Tetrahedron Lett., 1973, 14(45): 4519-4522. |
| [7] | Semmelhack M F, Chong B P, Stauffer R D, Rogerson T D, Chong A, Jones L D. Total synthesis of the Cephalotaxus alkaloids. Problem in nucleophilic aromatic substitution[J]. J. Am. Chem. Soc., 1975, 97(9): 2507-2516. |
| [8] | Satoh T, Kawamura Y, Miura M, Nomura M. Palladium-catalyzed regioselective mono- and diarylation reactions of 2-phenylphenols and naphthols with aryl halides[J]. Angew. Chem. Int. Ed. Engl., 1997, 36(16): 1740-1742. |
| [9] | Palucki M, Buchwald S L. Palladium-catalyzed α-arylation of ketones[J]. J. Am. Chem. Soc., 1997, 119(45): 11108-11109. |
| [10] | Hamann B C, Hartwig J F. Palladium-catalyzed direct α-arylation of ketones. rate acceleration by sterically hindered chelating ligands and reductive elimination from a transition metal enolate complex[J]. J. Am. Chem. Soc., 1997, 119(50): 12382-12383. |
| [11] | Matsubara K, Ueno K, Koga Y, Hara K. Nickel-NHC-catalyzed α-arylation of acyclic ketones and amination of haloarenes and unexpected preferential N-Arylation of 4-aminopropiophenone[J]. J. Org. Chem., 2007, 72(14): 5069-5076. |
| [12] | Terrett J A, Cuthbertson J D, Shurtleff V W, MacMillan D W C. Switching on elusive organometallic mechanisms with photoredox catalysis[J]. Nature, 2015, 524: 330-334. |
| [13] | Liu D, Ma H X, Fang P, Mei T S. Nickel-catalyzed thiolation of aryl halides and heteroaryl halides through electrochemistry[J]. Angew. Chem. Int. Ed., 2019, 58(15): 5033-5037. |
| [14] | Liu D, Liu Z R, Ma C, Jiao K J, Sun B, Wei L, Lefranc J, Herbert S, Mei T S. Nickel-catalyzed N-arylation of NH-sulfoximines with aryl halides via paired electrolysis[J]. Angew. Chem. Int. Ed., 2021, 60(17): 9444-9449. |
| [15] | Wei L, Wang Z H, Jiao K J, Liu D, Ma C, Fang P, Mei T S. Esterification of carboxylic acids with aryl halides via the merger of paired electrolysis and nickel catalysis[J]. J. Org. Chem., 2021, 86(22): 15906-15913. |
| [16] | Wang Z H, Wei L, Jiao K J, Ma C, Mei T S. Nickel-catalyzed decarboxylative cross-coupling of indole-3-acetic acids with aryl bromides by convergent paired electrolysis[J]. Chem. Commun., 2022, 58(59): 8202-8205. |
| [17] | Liu D, Liu Z R, Wang Z H, Ma C, Herbert S, Schirok H, Mei T S. Paired electrolysis-enabled nickel-catalyzed enantioselective reductive cross-coupling between α-chloroesters and aryl bromides[J]. Nat. Commun., 2022, 13: 7318. |
| [18] | Espinoza E M, Clark J A, Soliman J, Derr J B, Morales M, Vullev V I. Practical aspects of cyclic voltammetry: How to estimate reduction potentials when irreversibility prevails[J]. J. Electrochem. Soc., 2019, 166(5): H3175-H3187. |
| [19] | Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Petersson G A, Nakatsuji H, Li X, Caricato M, Marenich A V, Bloino J, Janesko B G, Gomperts R, Mennucci B, Hratchian H P, Ortiz J V, Izmaylov A F, Sonnenberg J L, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski V G, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery J A, Jr, Peralta J E, Ogliaro F, Bearpark M J, Heyd J J, Brothers E N, Kudin K N, Staroverov V N, Keith T A, Kobayashi R, Normand J, Raghavachari K, Rendell A P, Burant J C, Iyengar S S, Tomasi J, Cossi M, Millam J M, Klene M, Adamo C, Cammi R, Ochterski J W, Martin R L, Morokuma K, Farkas O, Foresman J B, Fox D J. Gaussian 16[CP]. Revision A.03. Wallingford, CT: Gaussian, Inc., 2016. |
| [20] | Adamo C, Barone V. Toward reliable density functional methods without adjustable parameters: The PBE0 model[J]. J. Chem. Phys., 1999, 110: 6158-6170. |
| [21] | Grimme S, Antony J, Ehrlich S, Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. J. Chem. Phys., 2010, 132: 154104. |
| [22] | Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory[J]. J. Comput. Chem., 2011, 32(7): 1456-1465. |
| [23] | Marenich A V, Cramer C J, Truhlar D G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions[J]. J. Phys. Chem. B, 2009, 113(18): 6378-6396. |
| [24] | Weigenda F, Ahlrichsb R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy[J]. Phys. Chem. Chem. Phys., 2005, 7(18): 3297-3305. |
| [25] | Weigenda F. Accurate coulomb-fitting basis sets for H to Rn[J]. Phys. Chem. Chem. Phys., 2006, 8(9): 1057-1065. |
| [26] | Legault C Y. CYLView[CP]. 1.0b, Sherbrooke, QC: Université de Sherbrooke, http://www.cylview.org ; 2009. |
| [27] | Li Y H. Energy diagram plotter CDXML[CP]. 3.5.2, Zenodo, 2023, https://doi.org/10.5281/zenodo.7634466. |
| [28] | Lu T, Chen F. Multiwfn: A multifunctional wavefunction analyzer[J]. J. Comput. Chem., 2012, 33(5): 580-592. |
| [29] | Lu T, Chen Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems[J]. J. Comput. Chem., 2022, 43(8): 539-555. |
| [30] | Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics[J]. J. Molec. Graphics, 1996, 14(1): 33-38. |
| [31] | Stone J E. An efficient library for parallel ray tracing and animation[D]. Missouri, USA: University of Missouri-Rolla, 1998. |
| [32] | Oka N, Yamada T, Sajiki H, Akai S, Ikawa T. Aryl boronic esters are stable on silica gel and reactive under Suzuki-Miyaura coupling conditions[J]. Org. Lett., 2022, 24(19): 3510-3514. |
| [33] | Haynes W M, Lide D R, Bruno T J. CRC Handbook of Chemistry and Physics[M]. 97th ed. Boca Raton, USA: CRC Press, 2016. 6-199-6-220. |
| [34] | Kawamata Y, Vantourout J C, Hickey D P, Bai P, Chen L, Hou Q, Qiao W, Barman K, Edwards M A, Garrido-Castro A F, deGruyter J N, Nakamura H, Knouse K, Qin C, Clay K J, Bao D, Li C, Starr J T, Garcia-Irizarry C, Sach N, White H S, Neurock M, Minteer S D, Baran P S. Electrochemically driven, Ni-catalyzed aryl amination: scope, mechanism, and applications[J]. J. Am. Chem. Soc., 2019, 141(15): 6392-6402. |
| [35] | Till N A, Oh S, MacMillan D W C, Bird M J. The application of pulse radiolysis to the study of Ni(I) intermediates in Ni-catalyzed cross-coupling reactions[J]. J. Am. Chem. Soc., 2021, 143(25): 9332-9337. |
| [36] | Gutierrez O, Tellis J C, Primer D N, Molander G A, Kozlowski M C. Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings[J]. J. Am. Chem. Soc., 2015, 137(15): 4896-4899. |
| [37] | Yakhvarov D G, Samieva E G, Tazeev D I, Budnikovaa Y G. The reactivity of 2,2′-bipyridine complexes in the electrochemical reduction of organohalides[J]. Russ. Chem. Bull., Int. Ed., 2002, 51: 796-804. |
| [38] | Diccianni J, Lin Q, Diao T. Mechanisms of nickel-catalyzed coupling reactions and applications in alkene functionalization[J]. Acc. Chem. Res., 2020, 53(4): 906-919. |
/
| 〈 |
|
〉 |