

机器学习加速氧化还原电位和酸度常数计算
收稿日期: 2023-07-18
修回日期: 2023-10-04
录用日期: 2023-11-06
网络出版日期: 2023-11-06
基金资助
国家自然科学基金项目(22225302);国家自然科学基金项目(21991151);国家自然科学基金项目(21991150);国家自然科学基金项目(22021001);国家自然科学基金项目(92161113);中央高校基本科研业务费专项资金(20720220009);AI4EC联合实验室基金(RD2023100101);AI4EC联合实验室基金(RD2022070501)
Automated Workflow for Redox Potentials and Acidity Constants Calculations from Machine Learning Molecular Dynamics
Received date: 2023-07-18
Revised date: 2023-10-04
Accepted date: 2023-11-06
Online published: 2023-11-06
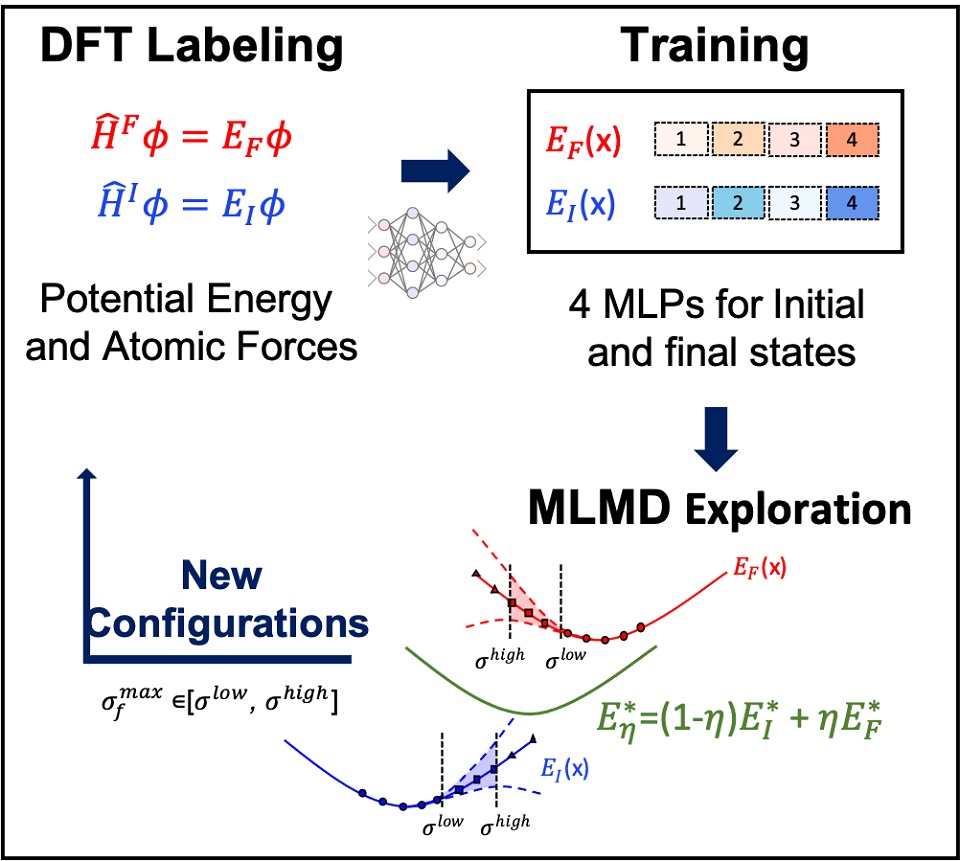
氧化还原电位和酸度常数作为重要的物理化学性质被应用于分析能源材料重要指标值。为了实现能源材料的计算设计,发展计算电化学的方法,在复杂电化学环境下计算这些性质至关重要。近年来,利用计算电化学方法计算氧化还原电位和酸度常数已经受到了广泛的关注。然而,常用的计算方法如基于隐式溶剂化模型的小分子自由能计算,对于复杂溶剂化环境的处理非常有限。因此,基于第一性原理分子动力学(AIMD)的自由能计算被引入来描述复杂溶剂化环境中的溶质-溶剂相互作用。同时,基于AIMD的自由能计算方法已经被证实可以准确预测这些物理化学性质。然而,由于AIMD计算效率低且计算资源需求大,需要引入机器学习分子动力学(MLMD)加速计算。MLMD通过机器学习方法,构建模拟体系结构到第一性原理计算结果的一对一映射,可以在低成本下实现长时间尺度的AIMD。对于氧化还原电位和酸度常数计算,如何构建训练机器学习势函数模型所需的数据集至关重要。本文介绍了如何通过自动化工作流实现自由能计算势函数的自动化构建,通过机器学习分子动力学计算自由能并转化为对应的物理化学性质。
王锋 , 程俊 . 机器学习加速氧化还原电位和酸度常数计算[J]. 电化学, 2024 , 30(2) : 2307181 . DOI: 10.13208/j.electrochem.2307181
Redox potentials and acidity constants are key properties for evaluating the performance of energy materials. To achieve computational design of new generation of energy materials with higher performances, computing redox potentials and acidity constants with computational chemistry have attracted lots of attention. However, many works are done by using implicit solvation models, which is difficult to be applied to complex solvation environments due to hard parameterization. Recently, ab initio molecular dynamics (AIMD) has been applied to investigate real electrolytes with complex solvation. Furthermore, AIMD based free energy calculation methods have been established to calculate these physical chemical properties accurately. However, due to the low efficiency of ab initio calculations and the high computational costs, AIMD based free energy calculations are limited to systems with less than 1000 atoms. To solve the dilemma, machine learning molecular dynamics (MLMD) is introduced to accelerate the calculations. By using machine learning method to construct one-to-one mapping from structures to computed potential energies and atomic forces, molecular dynamics can be carried out with much low costs under ab initio accuracy. In order to achieve the MLMD based free energy calculation, a new scheme for machine learning potential (MLP) should be introduced to collect training datasets. By combining the free energy perturbation sampling method and concurrent learning scheme, the training datasets can be collected along the reaction’s pathway (insertion of an electron or a proton) with high efficiency and the free energy calculations based on MLMD show good accuracy in comparison with AIMD simulation. This paper describes how to constructing machine learning potential for free energy calculation through the automated workflow, and how to use MLMD to compute accurate free energy differences and corresponding physical chemical properties.

| [1] | Blumberger J. Recent advances in the theory and molecular simulation of biological electron transfer reactions[J]. Chem. Rev., 2015, 115(20): 11191-11238. |
| [2] | Wardman P. Reduction potentials of one‐electron couples involving free radicals in aqueous solution[J]. J. Phys. Chem. Ref. Data, 1989, 18(4): 1637-1755. |
| [3] | Weinberg D R, Gagliardi C J, Hull J F, Murphy C F, Kent C A, Westlake B C, Meyer T J. Proton-coupled electron transfer[J]. Chem. Rev., 2012, 112(7): 4016-4093. |
| [4] | Yu J, Zhao T S, Pan D. Tuning the performance of aqueous organic redox flow batteries via first-principles calculations[J]. J. Phys. Chem. Lett., 2020, 11(24): 10433-10438. |
| [5] | Tomasi J, Mennucci B, Cammi R. Quantum mechanical continuum solvation models[J]. Chem. Rev., 2005, 105(8): 2999-3094. |
| [6] | Leung K. Predicting the voltage dependence of interfacial electrochemical processes at lithium-intercalated graphite edge planes[J]. Phys. Chem. Chem. Phys., 2015, 17(3): 1637-1643. |
| [7] | Le J, Iannuzzi M, Cuesta A, Cheng J. Determining potentials of zero charge of metal electrodes versus the standard hydrogen electrode from density-functional-theory-based molecular dynamics[J]. Phys. Rev. Lett., 2017, 119(1): 016801. |
| [8] | Rossmeisl J, Skúlason E, Bj?rketun M E, Tripkovic V, N?rskov J K. Modeling the electrified solid-liquid interface[J]. Chem. Phys. Lett., 2008, 466(1-3): 68-71. |
| [9] | Zhang C, Sprik M. Finite field methods for the supercell modeling of charged insulator/electrolyte interfaces[J]. Phys. Rev. B, 2016, 94(24): 245309. |
| [10] | King G, Warshel A. Investigation of the free energy functions for electron transfer reactions[J]. J. Chem. Phys., 1990, 93(12): 8682-8692. |
| [11] | Cheng J, Sulpizi M, Sprik M. Redox potentials and pKa for benzoquinone from density functional theory based molecular dynamics[J]. J. Chem. Phys., 2009, 131(15): 154504. |
| [12] | Blumberger J, Tavernelli I, Klein M L, et al. Diabatic free energy curves and coordination fluctuations for the aqueous Ag+/Ag2+ redox couple: A biased born-oppenheimer molecular dynamics investigation[J]. J Chem. Phys., 2006, 124(6): 064507. |
| [13] | Costanzo F, Sulpizi M, Valle R G D, Sprik M. The oxidation of tyrosine and tryptophan studied by a molecular dynamics normal hydrogen electrode[J]. J. Chem. Phys., 2011, 134(24): 06B615. |
| [14] | Cheng J, Liu X, VandeVondele J, Sulpizi M, Sprik M. Redox potentials and acidity constants from density functional theory based molecular dynamics[J]. Acc. Chem. Res., 2014, 47(12): 3522-3529. |
| [15] | Yang X H, Cuesta A, Cheng J. Computational Ag/AgCl reference electrode from density functional theory-based molecular dynamics[J]. J. Phys. Chem. B, 2019, 123(48): 10224-10232. |
| [16] | Leung K, Tenney C M. Toward first principles prediction of voltage dependences of electrolyte/electrolyte interfacial processes in lithium ion batteries[J]. J. Phys. Chem. C, 2013, 117(46): 24224-24235. |
| [17] | Cheng J, VandeVondele J. Calculation of electrochemical energy levels in water using the random phase approximation and a double hybrid functional[J]. Phys. Rev. Lett., 2016, 116(8): 086402. |
| [18] | Su N Q, Xu X. The XYG3 type of doubly hybrid density functionals[J]. WIRES. Co. Mol. Sci., 2016, 6(6): 721-747. |
| [19] | Su N Q, Zhu Z, Xu X. Doubly hybrid density functionals that correctly describe both density and energy for atoms[J]. P. Natl. Acad. Sci., 2018, 115(10): 2287-2292. |
| [20] | Zhang I Y, Wu J, Xu X. Accurate heats of formation of polycyclic saturated hydrocarbons predicted by using the XYG3 type of doubly hybrid functionals[J]. J. Comput. Chem., 2019, 40(10): 1113-1122. |
| [21] | Thompson A P, Swiler L P, Trott C R, Foiles S M, Tucker G J. Spectral neighbor analysis method for automated generation of quantum-accurate interatomic potentials[J]. J. Comput. Phys., 2015, 285: 316-330. |
| [22] | Huan T D, Batra R, Chapman J, Krishnan S, Chen L, Ramprasad R. A universal strategy for the creation of machine learning-based atomistic force fields[J]. NPJ Comput. Mater., 2017, 3(1): 1-8. |
| [23] | Behler J, Parrinello M. Generalized neural-network representation of high-dimensional potential-energy surfaces[J]. Phys. Rev. Lett., 2007, 98(14): 146401. |
| [24] | Behler J. Perspective: Machine learning potentials for atomistic simulations[J]. J. Chem. Phys., 2016, 145(17): 170901. |
| [25] | Bartók A P, Payne M C, Kondor R, Csányi G. Gaussian approximation potentials: The accuracy of quantum mechanics, without the electrons[J]. Phys. Rev. Lett., 2010, 104(13): 136403. |
| [26] | Rupp M, Tkatchenko A, Müller K R, Von Lilienfeld O A. Fast and accurate modeling of molecular atomization energies with machine learning[J]. Phys. Rev. Lett., 2012, 108(5): 058301. |
| [27] | Zhang L, Han J, Wang H, Car R, Weinan E. Deep potential molecular dynamics: a scalable model with the accuracy of quantum mechanics[J]. Phys. Rev. Lett., 2018, 120(14): 143001. |
| [28] | Wang H, Zhang L, Han J, Weinan E. DeePMD-kit: A deep learning package for many-body potential energy representation and molecular dynamics[J]. Comput. Phys. Commun., 2018, 228: 178-184. |
| [29] | Chmiela S, Tkatchenko A, Sauceda H E, Poltavsky I, Schütt K T, Müller K R. Machine learning of accurate energy-conserving molecular force fields[J]. Sci. Adv., 2017, 3(5): e1603015. |
| [30] | Schütt K T, Arbabzadah F, Chmiela S, Müller K R, Tkatchenko A. Quantum-chemical insights from deep tensor neural networks[J]. Nat. Commun., 2017, 8(1): 1-8. |
| [31] | Wang F, Cheng J. Automated workflow for computation of redox potentials, acidity constants, and solvation free energies accelerated by machine learning[J]. J. Chem. Phys. 2022; 157(2): 024103 |
| [32] | Zhang L, Lin D Y, Wang H, Car R, Weinan E. Active learning of uniformly accurate interatomic potentials for materials simulation[J]. Phys. Rev. Mater., 2019, 3(2): 023804. |
| [33] | Zhang Y, Wang H, Chen W, Zeng J, Zhang L, Wang H, Weinan E. DP-GEN: A concurrent learning platform for the generation of reliable deep learning based potential energy models[J]. Comput. Phys. Commun., 2020, 253: 107206. |
| [34] | Kirkwood J G. Statistical mechanics of fluid mixtures[J]. J. Chem. Phys., 1935, 3(5): 300-313. |
| [35] | Sulpizi M, Sprik M. Acidity constants from vertical energy gaps: density functional theory based molecular dynamics implementation[J]. Phys. Chem. Chem. Phys., 2008, 10(34): 5238-5249. |
| [36] | Sun Y, Wu C R, Wang F, Bi R H, Zhuang Y B, Liu S, Cheng J. Step-induced double-row pattern of interfacial water on rutile TiO2 (110) at electrochemical conditions[J]. doi: 10.26434/chemrxiv-2023-7wsqv 2023. |
| [37] | VandeVondele J, Krack M, Mohamed F, Parrinello M, Chassaing T, Hutter J. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach[J]. Comput. Phys. Commun., 2005, 167(2): 103-128. |
| [38] | Becke A D. Density-functional exchange-energy approximation with correct asymptotic behavior[J]. Phys. Rev. A, 1988, 38(6): 3098. |
| [39] | Lee C, Yang W, Parr R G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density[J]. Phys. Rev. B, 1988, 37(2): 785. |
| [40] | Grimme S, Antony J, Ehrlich S & Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. J. Chem. Phys., 2010, 132(15): 154104. |
| [41] | Hartwigsen C, G?decker S, Hutter J. Relativistic separable dual-space Gaussian pseudopotentials from H to Rn[J]. Phys. Rev. B, 1998, 58(7): 3641. |
| [42] | Goedecker S, Teter M, Hutter J. Separable dual-space Gaussian pseudopotentials[J]. Phys. Rev. B, 1996, 54(3): 1703. |
| [43] | VandeVondele J, Hutter J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases[J]. J. Chem. Phys., 2007, 127(11): 114105. |
| [44] | Zhang L, Han J, Wang H, Saidi W & Car R. End-to-end symmetry preserving inter-atomic potential energy model for finite and extended systems[J]. Adv. Neural Inf. Process. Syst., 2018: 31. |
| [45] | Sulpizi M, Sprik M. Acidity constants from vertical energy gaps: density functional theory based molecular dynamics implementation[J]. Phys. Chem. Chem. Phys., 2008, 10(34): 5238-5249. |
/
| 〈 |
|
〉 |