

电催化活性亚甲基化合物的环丙烷化反应
收稿日期: 2023-03-22
修回日期: 2023-05-11
录用日期: 2023-06-29
网络出版日期: 2023-07-11
Electrocatalytic Cyclopropanation of Active Methylene Compounds
Received date: 2023-03-22
Revised date: 2023-05-11
Accepted date: 2023-06-29
Online published: 2023-07-11
揭亮华 , 徐海超 . 电催化活性亚甲基化合物的环丙烷化反应[J]. 电化学, 2024 , 30(4) : 2313001 . DOI: 10.13208/j.electrochem.2313001
The development of novel strategies to access cyclopropanes has become increasingly important due to the vital role of these three-membered ring structures in synthetic intermediates, natural products, and pharmaceuticals. Herein, we present an electrocatalytic method for the synthesis of cyclopropanes through intermolecular dehydrogenative annulation of active methylene compounds and arylalkenes. This electrochemical process requires no chemical oxidants, allowing for a speedy access to various functionalized cyclopropanes from inexpensive and readily available materials.
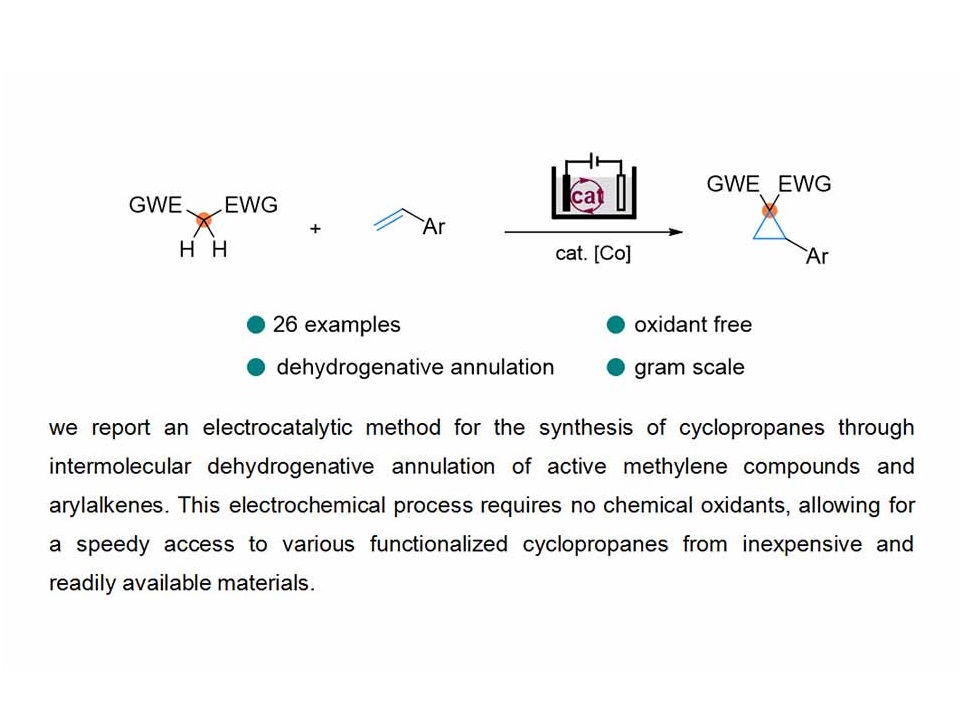
Key words: Electrochemistry; Cyclopropane; Catalyst; Alkene; Organic electrosynthesis
| [1] | Pirenne V, Muriel B, Waser J V. Catalytic enantioselective ring-opening reactions of cyclopropanes[J]. Chem. Rev., 2021, 121(1): 227-263. |
| [2] | Liu J X, Liu R X, Wei Y, Shi M. Recent developments in cyclopropane cycloaddition reactions[J]. Trends Chem., 2019, 1(8): 779-793. |
| [3] | Ebner C, Carreira E M. Cyclopropanation strategies in recent total syntheses[J]. Chem. Rev., 2017, 117(18): 11651-11679. |
| [4] | Talele T T. The "cyclopropyl fragment" is a versatile player that frequently appears in preclinical/clinical drug molecules[J]. J. Med. Chem., 2016, 59(19): 8712-8756. |
| [5] | Chen D Y K, Pouwer R H, Richard J A. Recent advances in the total synthesis of cyclopropane-containing natural products[J]. Chem. Soc. Rev., 2012, 41(13): 4631-4642. |
| [6] | Zheng Z B, Cheng W F, Wang L J, Zhu J, Sun X L, Tang Y. Asymmetric catalytic [3+2] annulation ofdonor-acceptorcyclopropane with cyclic ketones: Facile access to enantioenriched1-oxaspiro[4.5]decanes[J]. Chin. J. Chem., 2020, 38(12): 1629-1634. |
| [7] | Bi X F, Zhang Q C, Gu Z H. Transition-metal-catalyzed carbon-carbon bond activation in asymmetric synthesis[J]. Chin. J. Chem., 2021, 39(5): 1397-1412. |
| [8] | Ford A, Miel H, Ring A, Slattery C N, Maguire A R, McKervey M A. Modern organic synthesis with alpha-diazocarbonyl compounds[J]. Chem. Rev., 2015, 115(18): 9981-10080. |
| [9] | Maas G. Ruthenium-catalysed carbenoid cyclopropanation reactions with diazo compounds[J]. Chem. Soc. Rev., 2004, 33(3): 183-190. |
| [10] | Ouyang Y Z, Zhan M, Zhou J, Jiao J, Hao H U, Yamada Y M A, Li P F. Z-bpy,a new c2-symmetric bipyridine ligand and its application in enantioselective copper (I)-catalyzed cyclopropanation of olefins[J]. Chin. J. Chem., 2019, 37(8): 807-810. |
| [11] | Green S P, Wheelhouse K M, Payne A D, Hallett J P, Miller P W, Bull J A. Thermal stability and explosive hazard assessment of diazo compounds and diazo transfer reagents[J]. Org. Process Res. Dev., 2020, 24(1): 67-84. |
| [12] | Schilter D. Doing without diazos[J]. Nat. Catal., 2021, 4(5): 347-347. |
| [13] | Jia M Q, Ma S M. New approaches to the synthesis of metal carbenes[J]. Angew. Chem. Int. Ed., 2016, 55(32): 9134-9166. |
| [14] | Ye L W, Zhu X Q, Sahani R L, Xu Y, Qian P C, Liu R S. Nitrene transfer and carbene transfer in gold catalysis[J]. Chem. Rev., 2021, 121(14): 9039-9112. |
| [15] | Zhang L. A non-diazo approach to α-oxo gold carbenes via gold-catalyzed alkyne oxidation[J]. Acc. Chem. Res., 2014, 47(3): 877-888. |
| [16] | Zhu D, Chen L F, Fan H L, Yao Q L, Zhu S F. Recent progress on donor and donor-donor carbenes[J]. Chem. Soc. Rev., 2020, 49(3): 908-950. |
| [17] | Moreau B, Charette A B. Expedient synthesis of cyclopropane alpha-amino acids by the catalytic asymmetric cyclopropanation of alkenes using iodonium ylides derived from methyl nitroacetate[J]. J. Am. Chem. Soc., 2005, 127(51): 18014-18015. |
| [18] | Cao L Y, Luo J N, Yao J S, Wang D K, Dong Y Q, Zheng C, Zhuo C X. Molybdenum-catalyzed deoxygenative cyclopropanation of 1,2-dicarbonyl or monocarbonyl compounds[J]. Angew. Chem. Int. Ed., 2021, 60(28): 15254-15259. |
| [19] | Fischer D M, Lindner H, Amberg W M, Carreira E M. Intermolecular organophotocatalytic cyclopropanation of unactivated olefins[J]. J. Am. Chem. Soc., 2023, 145(2): 774-780. |
| [20] | Yuan Y, Yang J, Lei A W. Recent advances in electrochemical oxidative cross-coupling with hydrogen evolution involving radicals[J]. Chem. Soc. Rev., 2021, 50(18): 10058-10086. |
| [21] | Cheng X, Lei A, Mei T S, Xu H C, Xu K, Zeng C. Recent applications of homogeneous catalysis in electrochemical organic synthesis[J]. CCS Chem., 2022, 4: 1120-1152. |
| [22] | Jie L H, Guo B, Song J S, Xu H C. Organoelectrocatalysis enables direct cyclopropanation of methylene compounds[J]. J. Am. Chem. Soc., 2022, 144(5): 2343-2350. |
| [23] | Xiong P, Xu H C. Chemistry with electrochemically generated N-centered radicals[J]. Acc. Chem. Res., 2019, 52(12): 3339-3350. |
| [24] | Zhu L, Xiong P, Mao Z Y, Wang Y H, Yan X M, Lu X, Xu H C. Electrocatalytic generation of amidyl radicals for olefin hydroamidation: Use of solvent effects to enable anilide oxidation[J]. Angew. Chem. Int. Ed., 2016, 55(6): 2226-2229. |
| [25] | Hou Z W, Yan H, Song J S, Xu H C. Electrochemical synthesis of (Aza)indolines via dehydrogenative [3+2] annulation: application to total synthesis of (±)-hinckdentine A?[J]. Chin. J. Chem., 2018, 36(10): 909-915. |
| [26] | Yan H, Hou Z W, Xu H C. Photoelectrochemical C-H alkylation of heteroarenes with organotrifluoroborates[J]. Angew. Chem. Int. Ed., 2019, 58(14): 4592-4595. |
| [27] | Huang C, Qian X Y, Xu H C. Continuous-flow electrosynthesis of benzofused S-heterocycles by dehydrogenative C-S cross-coupling[J]. Angew. Chem. Int. Ed., 2019, 58(20): 6650-6653. |
| [28] | Cai C Y, Lai X L, Wang Y, Hu H H, Song J, Yang Y, Wang C, Xu H C. Photoelectrochemical asymmetric catalysis enables site- and enantioselective cyanation of benzylic C-H bonds[J]. Nat. Catal., 2022, 5(10): 943-951. |
| [29] | Yan H, Song J, Zhu S, Xu H C. Synthesis of acridinium photocatalysts via site-selective C-H alkylation[J]. CCS Chem., 2021, 3: 317-325. |
| [30] | Liu C K, Lin Y, Cai C, Yuan C C, Fang Z, Guo K. Continuous-flow electro-oxidative coupling of sulfides with activated methylene compounds leading to sulfur ylides[J]. Green Chem., 2021, 23(8): 2956-2961. |
| [31] | Chen M, Wu Z J, Song J, Xu H C. Electrocatalytic allylic C-H alkylation enabled by a dual-function cobalt catalyst[J]. Angew. Chem. Int. Ed., 2022, 61(14): e202115954. |
| [32] | Cai C Y, Wu Z J, Liu J Y, Chen M, Song J, Xu H C. Tailored cobalt-salen complexes enable electrocatalytic intramolecular allylic C-H functionalizations[J]. Nat. Commun., 2021, 12(1): 3745. |
| [33] | Qin T, Lv G, Mia H, Guan M, Xu C, Zhang G, Xiong T, Zhang Q. Cobalt-catalyzed asymmetric alkylation of (hetero)arenes with styrenes[J]. Angew. Chem. Int. Ed., 2022, 61(26): e202201967. |
| [34] | Yin Y N, Ding R Q, Ouyang D C, Zhang Q, Zhu R. Highly chemoselective synthesis of hindered amides via cobalt-catalyzed intermolecular oxidative hydroamidation[J]. Nat. Commun., 2021, 12(1): 2552. |
| [35] | Ebisawa K, Izumi K, Ooka Y, Kato H, Kanazawa S, Komatsu S, Nishi E, Shigehisa H. Catalyst- and silane-controlled enantioselective hydrofunctionalization of alkenes by cobalt-catalyzed hydrogen atom transfer and radical-polar crossover[J]. J. Am. Chem. Soc., 2020, 142(31): 13481-13490. |
/
| 〈 |
|
〉 |