

用纳米羟基磷灰石@多孔碳构建锂硫电池高效反应界面
收稿日期: 2022-09-21
修回日期: 2022-11-04
网络出版日期: 2022-11-28
Efficient Interface Enabled by Nano-Hydroxyapatite@Porous Carbon for Lithium-Sulfur Batteries
Received date: 2022-09-21
Revised date: 2022-11-04
Online published: 2022-11-28
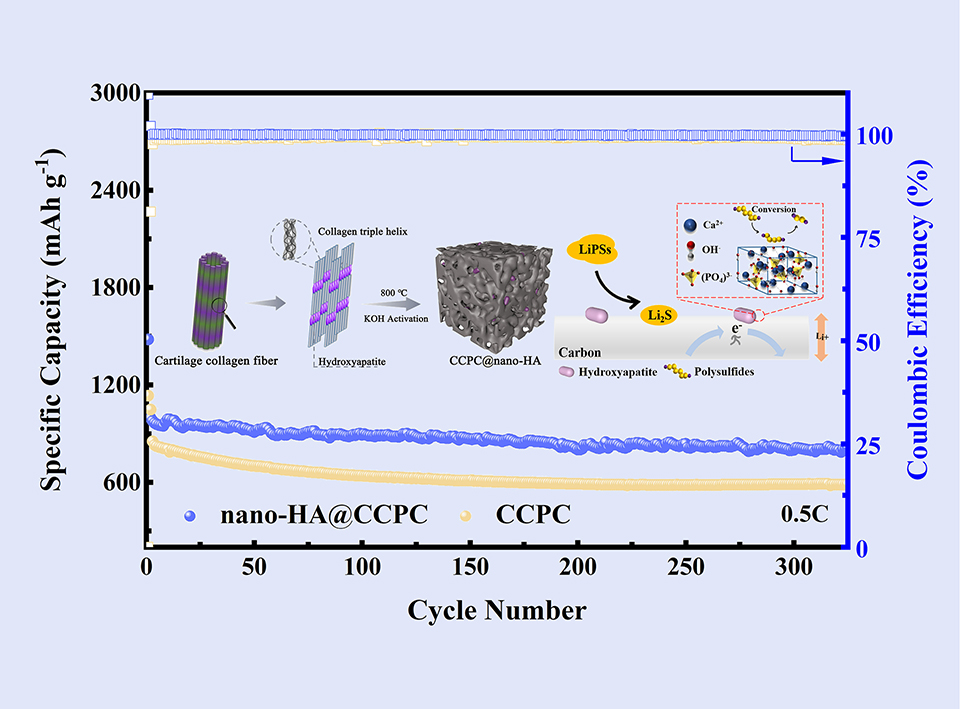
由于正极活性物质硫具有能量密度高、成本低廉和储量丰富等优点,锂硫(Li-S)电池受到了人们的极大关注。然而,锂硫电池充放电过程中产生的多硫化锂的“穿梭效应”严重阻碍了其实用化进程。为了解决这个问题,本研究借助动物软骨的组成和结构特点,制备了纳米羟基磷灰石@多孔碳(nano-HA@CCPC)复合材料,并以此设计了面向正极的锂硫电池隔膜涂层。研究表明,纳米羟基磷灰石不仅对多硫化物具有吸附固定作用,并且对多硫化锂的转化具有催化作用,加快了多硫化锂的氧化还原动力学,有效地提升了活性物质硫的利用率。另外,软骨基碳复合材料的多孔结构形成了很好的导电网络,为电化学反应提供了优良的电子传导路径;也有利于电解液的浸润,加快了离子传输;碳的氮原子掺杂进一步限制了多硫化物的穿梭效应。因此,采用nano-HA@CCPC隔膜涂层的锂硫电池表现出较长的循环寿命、低的容量损失以及高的倍率性能。在0.5 C下,循环325次后,电池仍然能保持815 mAh·g-1的放电比容量,并且每次的容量衰减率仅为0.051%。nano-HA@CCPC的设计制备将为锂硫电池的发展提供新材料。
汪佳裕 , 仝学锋 , 彭启繁 , 关越鹏 , 王维坤 , 王安邦 , 刘乃强 , 黄雅钦 . 用纳米羟基磷灰石@多孔碳构建锂硫电池高效反应界面[J]. 电化学, 2022 , 28(11) : 2219008 . DOI: 10.13208/j.electrochem.2219008
The dissolution and “shuttle effect” of lithium polysulfides (LiPSs) hinder the application of lithium-sulfur (Li-S) batteries. To solve those problems, inspired by natural materials, a nano-hydroxyapatite@porous carbon derived from chicken cartilage (nano-HA@CCPC) was fabricated by employing a simple pre-carbonization and carbonization method, and applied in Li-S batteries. The nano-HA@CCPC would provide a reactive interface that allows efficient LiPSs reduction. With a strong affinity for LiPSs and an excellent electronic conductive path for converting LiPSs, the shuttle effect of LiPSs was confined and the redox kinetics of LiPSs was substantially enhanced. Li-S batteries employing nano-HA@CCPC-modified separators exhibited long cycle life and improved rate capability. At 0.5 C after 325 cycles, a specific capacity of 815 mAh·g-1 and a low capacity fading rate of 0.051% were obtained. The superior properties, sustainable raw materials, and facile preparation process make nano-HA@CCPC a promising additive material for practical Li-S batteries.

/
| 〈 |
|
〉 |