

PCB酸性蚀刻液中缓蚀剂对厚铜线路制作的影响
收稿日期: 2022-03-04
修回日期: 2022-04-14
网络出版日期: 2022-05-17
Effect of Corrosion Inhibitors on Copper Etching to Form Thick Copper Line of PCB in Acidic Etching Solution
Received date: 2022-03-04
Revised date: 2022-04-14
Online published: 2022-05-17
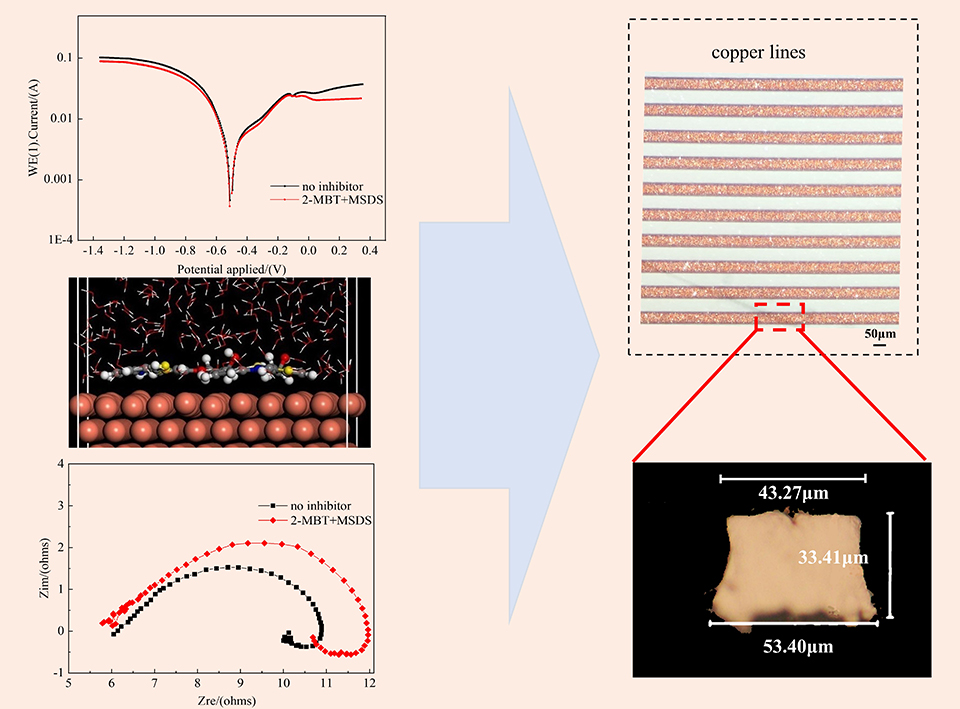
以2-巯基苯并噻唑(2-MBT)、 苯并三氮唑(BTA)和苯氧基乙醇(MSDS)作为缓蚀剂, 研究了其加入在酸性蚀刻液后对PCB厚铜线路的缓蚀效果。通过接触角测试、电化学测试和蚀刻因子得出缓蚀状态,并结合扫描电子显微镜观察铜表面形貌。通过分子动力学计算和量子化学模拟分析缓蚀剂在铜表面的吸附机理。结果表明,2-MBT + MSDS与BTA + MSDS的分子结构可有效地平行吸附在铜表面,且吸附能高于单一缓蚀剂。加入了2-MBT + MSDS的蚀刻液,对厚度约为33 μm铜线路进行刻蚀,铜线路的蚀刻因子提高到6.59,可有效应用于PCB厚铜线路制作。
王小丽 , 何为 , 陈先明 , 曾红 , 苏元章 , 王翀 , 李高升 , 黄本霞 , 冯磊 , 黄高 , 陈苑明 . PCB酸性蚀刻液中缓蚀剂对厚铜线路制作的影响[J]. 电化学, 2022 , 28(7) : 2213007 . DOI: 10.13208/j.electrochem.2213007
The chemical compounds of 2-mercaptobenzothiazole (2-MBT), benzotriazole (BTA) and phenoxyethanol (MSDS) as corrosion inhibitors were used to inhibit the copper etching to form the thick copper line of PCB in the acidic etching solution. The inhibition status was characterized with contact angle measurement, electrochemical test and etch factor calculation, while the corrosion morphology of copper surface was studied by scanning electron microscope. The adsorption mechanism of corrosion inhibitors on copper surface is analyzed by molecular dynamics and quantum chemistry calculations. The results indicated that the synergistic function of the two inhibitors could effectively promote their adsorption on the copper surface in parallel, while their adsorption energy could be higher than that of the single inhibitor. The etch factor of the thick copper line with about 33 μm in thickness increased to 6.59 from the etching solution with 2-MBT and MSDS for good agreement of PCB manufacture.

| [1] | He W. Electrical information science and technology[M]. Beijing: China Machine Press, 2021. 1-11. |
| [2] | Huang H L, Guo X P, Zhang G A, Dong Z H. Effect of direct current electric field on atmospheric corrosion behavior of copper under thin electrolyte layer[J]. Corrosion Sci., 2011, 53(10): 3446-3449. |
| [3] | Zhang S T, Tao Z H, Li W H, Hou B R. The effect of some triazole derivatives as inhibitors for the corrosion of mild steel in 1 M hydrochloric acid[J]. Appl. Surf. Sci., 2009, 255(15): 6757-6763. |
| [4] | Papapanayiotou D, Deligianni H, Alkire R C. Effect of Benzotriazole on the anisotropic electrolytic etching of copper[J]. J. Electrochem. Soc., 1998, 145(9): 3016-3024. |
| [5] | Cakir O. Copper etching with cupric chloride and regeneration of waste etchant[J]. J. Mater. Process. Technol., 2006, 175(1-3): 63-68. |
| [6] | Chen Y M, He W, Chen X M, Wang C, Tao Z H, Wang S X, Zhou G Y, Moshrefi-Torbati M. Plating uniformity of bottom-up copper pillars and patterns for IC substrates with additive-assisted electrodeposition[J]. Electrochim. Acta, 2014, 120: 293-301. |
| [7] | Zhong Y Q, Zhang W F, Jin L K, Sun B H. Improvement of fine line manufacturing by etching addictive[J]. Printed Circuit Information, 2018, 15(2):56-62. |
| [8] | Guo X M, Huang H L, Liu D. The inhibition mechanism and adsorption behavior of three purine derivatives on the corrosion of copper in alkaline artificial seawater: structure and performance[J]. Colloid Surf. A-Physicochem. Eng. Asp., 2021, 622: 126644. |
| [9] | Li L, Zhang X H, Gong S D, Zhao H X, Bai Y, Li Q S, Ji L. The discussion of descriptors for the QSAR model and molecular dynamics simulation of benzimidazole derivatives as corrosion inhibitors[J]. Corrosion Sci., 2015, 99: 76-88. |
| [10] | Sherif E S M, Erasmus R M, Comins J D. Inhibition of copper corrosion in acidic chloride pickling solutions by 5-(3-aminophenyl)-tetrazole as a corrosion inhibitor[J]. Corrosion Sci., 2008, 50(12): 3439-3445. |
| [11] | Lei S S, Wang S L, Li H L, Wang C W, Yang Y D A, Cheng Y S, Li S. Effect of benzotriazole and 5-methyl/ 1-H carboxyl benzotriazole on chemical mechanical polishing of cobalt in H2O2 based slurry[J]. ECS J. Solid State Sci. Technol., 2021, 10(7): 074002. |
| [12] | Moretti G, Guidi F, Grion G. Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid[J]. Corrosion Sci., 2004, 46(2): 387-403. |
| [13] | Huang H L, Guo X M. The Relationship between the inhibition performances of three benzo derivatives and their structures on the corrosion of copper in 3.5wt.% NaCl solution[J]. Colloid Surf. A-Physicochem. Eng. Asp., 2020, 598: 124809. |
| [14] | Chiter F, Costa D, Maurice V, Marcus P. DFT investigation of 2-mercaptobenzothiazole adsorption on model oxidized copper surfaces and relationship with corrosion inhibition[J]. Appl. Surf. Sci., 2020, 537: 147802. |
| [15] | Li G S. Investigation on etching technology and corrosion inhibition mechanism of thick copper circuit[D]. Chengdu: University of Electronic Science and Technology of China, 2020. |
| [16] | Guo L, Dong W P, Zhang S T. Theoretical challenges in understanding the inhibition mechanism of copper corrosion in acid media in the presence of three triazole derivatives[J]. RSC Adv., 2014, 4(79): 41956-41967. |
| [17] | Gece G, Bilgi S, Türken. Quantum chemical studies of some amino acids on the corrosion of cobalt in sulfuric acid solution[J]. Mater. Corros., 2010, 61(2): 141-146. |
| [18] | Awad M K, Mustafa M R, Elnga M M A. Computational simulation of the molecular structure of some triazoles as inhibitors for the corrosion of metal surface[J]. Theochem-J. Mol. Struct., 2010, 959(1-3): 66-74. |
| [19] | Chen J, Qiang Y J, Peng S N, Gong Z L, Zhang S T, Gao L Z, Tan B C, Chen S J, Guo L. Experimental and computational investigations of 2-amino-6-bromobenzothiazole as a corrosion inhibitor for copper in sulfuric acid[J]. J. Adhes. Sci. Technol., 2018, 32(19): 2083-2098. |
| [20] | Bastidas J M, Pinilla P, Cano E, Polo J L, Miguel S. Copper corrosion inhibition by triphenylmethane derivatives in sulphuric acid media[J]. Corrosion Sci., 2003, 45(2): 427-449. |
| [21] | Tao Z H, He W, Wang S X, Zhou G Y. Electrochemical study of cyproconazole as a novel corrosion inhibitor for copper in acidic solution[J]. Ind. Eng. Chem. Res., 2013, 52(50): 17891-17899. |
| [22] | Aboelnga M M, Awad M K, Gauld J W, Mustafa M R. An assessment to evaluate the validity of different methods for the description of some corrosion inhibitors[J]. J. Mol. Model., 2014, 20(9): 2422. |
/
| 〈 |
|
〉 |