

析氧反应铁镍基预催化剂的表界面调控与进展
Surface Structure Engineering of FeNi-Based Pre-Catalyst for Oxygen Evolution Reaction: A Mini Review
Received date: 2022-04-06
Revised date: 2022-04-11
Online published: 2022-04-25
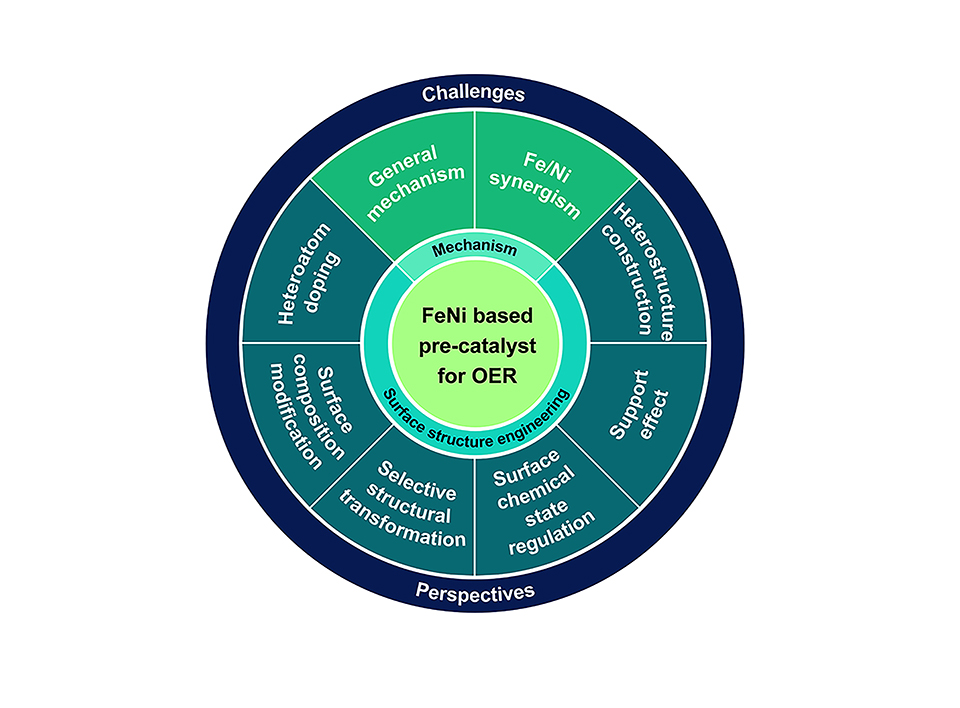
析氧反应(OER)是水分解中重要的半反应, 为提高其催化性能,开发高效非贵金属催化剂已成为当前的研究重点。铁镍(FeNi)基材料被认为是最好的预催化剂, 在催化过程中,它们的表面将转变成高价态金属氧化物或氢氧化物作为真正的活性物质。FeNi基预催化剂的结构和形貌在很大程度上影响了其催化性能, 因此, 优化和调整FeNi基预催化剂的结构和化学环境可以提高电催化性能。基于我们的研究工作, 我们撰写了FeNi基预催化剂的表面结构调控促进电化学析氧反应的研究进展。我们首先介绍了碱性OER的反应机理, 然后从杂原子掺杂、表面成分改性、选择性结构转变、表面化学状态调节、异质结构构建和载体效应等方面讨论了FeNi基预催化剂表面调控对析氧反应性能的影响。尽管在OER反应中FeNi都被认为转变成高价态的金属活性物质, Fe/Ni体系的表面结构、形貌和化学状态仍然能够显著影响其最终的催化性能, 即FeNi基预催化剂的性质会影响析氧反应的催化性能。通过精细设计并尽量提高Fe和Ni的协同作用将有利用提升氧析出的催化性能。我们希望本综述能够对FeNi基预催化剂的制备和表界面性质调控与电催化析氧反应性能的理解有所帮助。
李家欣 , 冯立纲 . 析氧反应铁镍基预催化剂的表界面调控与进展[J]. 电化学, 2022 , 28(9) : 2214001 . DOI: 10.13208/j.electrochem.2214001
Oxygen evolution reaction (OER) is a significant half-reaction for water splitting reaction, and attention is directed to the high-performance non-precious catalysts. Iron nickel (FeNi)-based material is considered as the most promising pre-catalyst, that will be transferred to the real active phase in the form of high valence state metal species. Even so, the catalytic performance is largely influenced by the structure and morphology of the FeNi pre-catalysts, and lots of work has been done to optimize and tune the structure and chemical environment of the FeNi- based pre-catalysts so as to increase the catalytic performance. Herein, based on our work, a mini review is proposed for the surface structure engineering of FeNi-based pre-catalyst for OER. The reaction mechanism of alkaline OER is firstly presented, and then the strategies in surface engineering of FeNi-based pre-catalyst for improving OER performance are discussed in terms of heteroatom doping, surface composition modification, selective structural transformation, surface chemical state regulation, heterostructure construction, and support effect. It can be concluded that the surface structure, morphology, and the chemical states of Fe/Ni in the system will significantly influence the final catalytic performance, though all of them were transferred into the active phase state of high valence state metal species. In other words, the catalytic performance of FeNi-based catalysts is also determined by the property of their pre-catalysts. To carefully design and maximize the synergistic effect of Fe and Ni is necessary to boost the catalytic performance. We hope this topic will be a good and timely complement to the study of FeNi-based catalysts for OER in the water-splitting technique.

/
| 〈 |
|
〉 |