

PEG水系电解液用于高性能锌碘双离子电池
收稿日期: 2021-10-26
修回日期: 2021-11-30
录用日期: 2021-12-19
网络出版日期: 2022-01-02
基金资助
国家自然科学基金面上项目(21673051);广东省科技厅国际合作项目(2019A050510043)
PEG-Water Electrolyte for High-Performance Zinc Iodine Dual-Ion Batteries
Received date: 2021-10-26
Revised date: 2021-11-30
Accepted date: 2021-12-19
Online published: 2022-01-02
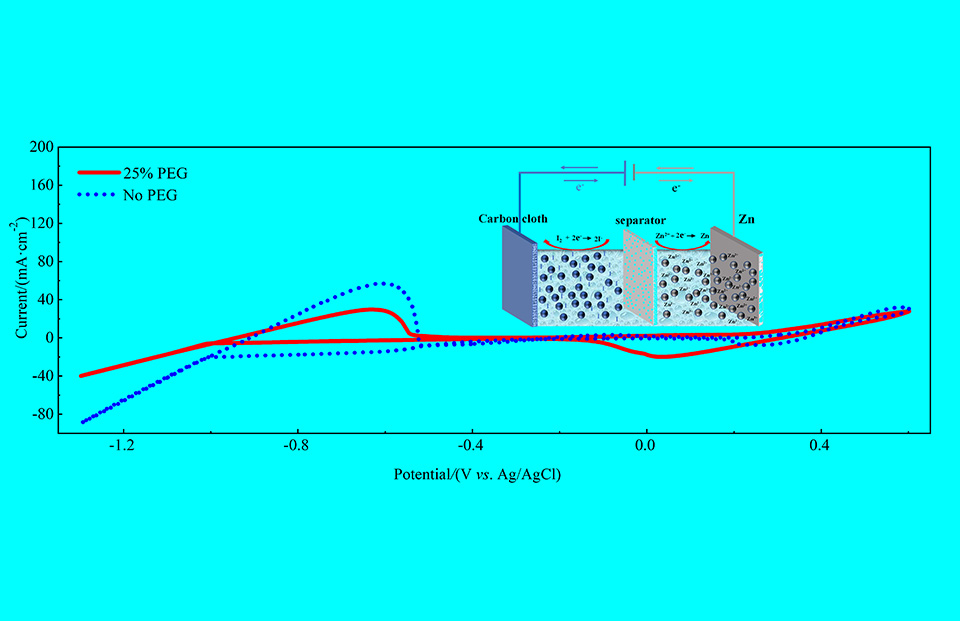
碘因来源丰富和具有较快的氧化还原反应动力学使其作为电池的正极材料而倍受青睐,然而,由于碘单质在电解液中的高溶解性而带来的穿梭效应,使得电池的性能下降。本文在水系锌离子电解液中添加聚乙二醇(PEG400)和碘化钾,PEG400能与碘发生络合,抑制了单质碘和碘离子生成碘三离子(I3-)的反应,进而避免了碘的溶解;最后,该电解液搭配双层碳布集流体、锌片及双层隔膜组装成电池,在1 mA·cm-2电流密度下,首圈容量可达1.62 mAh·cm-2,参与氧化还原反应碘占该电池电解液中碘质量的47.52%,库仑效率为93%左右;而在7 mA·cm-2高电流密度下,库仑效率可达98%左右,循环1200圈后,循环保持率为58.33%。
屈小峰 , 唐宇婷 , 何鑫程 , 周佳晟 , 唐子恒 , 冯文华 , 刘军 . PEG水系电解液用于高性能锌碘双离子电池[J]. 电化学, 2023 , 29(11) : 211026 . DOI: 10.13208/j.electrochem.211026
Thanks to abundant resource and rapid redox reaction kinetics, iodine is regarded as promising positive materials in the batteries. However, the shuttling effect due to the high solubility of iodine in the electrolyte makes the performance of battery poor. In this paper, polyethylene glycol (PEG400) and potassium iodide were added into zinc-ion aqueous electrolyte. PEG400 could complex with iodine to reduce the dissolution of iodine, therefore alleviating the formation of soluble triiodide (I3-) from iodine and iodide ions. Furthermore, this electrolyte was used in the battery with double carbon cloths as the current collectors, double separators and zinc anode. At the current density of 1 mA·cm-2, the first discharge capacity reached 1.62 mAh·cm-2, and the coulombic efficiency was around 93%. Iodine involved in the electrochemical redox reaction is calculated to account for 47.52% of the total mass of iodine in this electrolyte. At high current density of 7 mA·cm-2, its coulombic efficiency still remained 98%, and the rate of capacity retention was 58.33% after 1,200 cycles.

Key words: Zinc iodine battery; PEG400; Dual-ion batteries; Complexation; High current density
/
| 〈 |
|
〉 |