

碱性介质中非贵金属氧还原催化剂的结构调控进展
收稿日期: 2021-11-01
修回日期: 2021-12-02
网络出版日期: 2021-12-18
版权
Recent Advances in Structural Regulation on Non-Precious Metal Catalysts for Oxygen Reduction Reaction in Alkaline Electrolytes
Received date: 2021-11-01
Revised date: 2021-12-02
Online published: 2021-12-18
Copyright
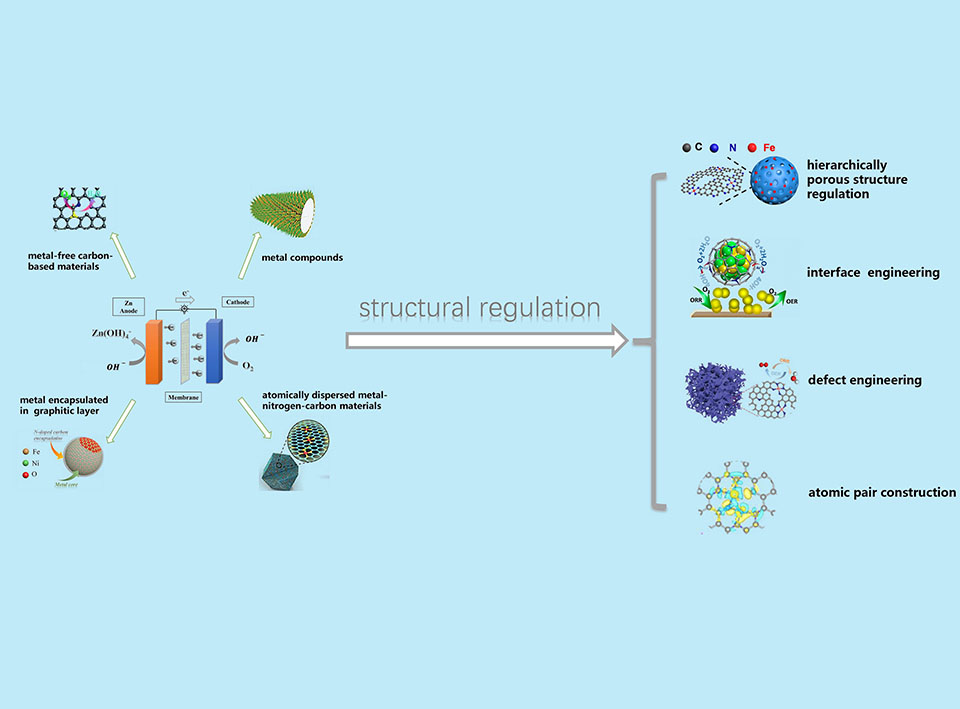
碱性介质中的氧还原反应是金属-空气电池和阴离子交换膜燃料电池的重要电化学过程。但是,其动力学缓慢,因而引起了对高效电催化剂的广泛研究。其中,非贵金属催化剂可有效地规避铂基催化剂成本和储量的问题,而备受关注。但其挑战在于将性能提高到可与Pt基催化材料媲美。鉴于非贵金属催化剂的组成和结构对催化性能有着至关重要的影响,精准地调控催化剂的结构有望消除非贵金属催化剂和商业铂基催化剂的活性差距。在该评述中,我们致力于总结通过结构调控来提升性能的研究进展。我们首先介绍了四种极具代表性的非贵金属催化剂,包括非金属碳基材料、金属化合物、石墨化碳层包覆金属颗粒、原子分散的金属-氮-碳材料,突出了催化活性位点和催化机理。随后,针对于这些催化剂,我们归纳了从微纳尺度到原子层面的结构调控策略,如分级多孔结构的设计、界面工程、缺陷工程以及原子对活性位点的构建。我们着重讨论了结构和性能之间的依赖关系。从加速传质、增加可及的活性位点数量、可调控的电子状态和多组分之间的协同效应,讨论了这些结构变化引起的活性改进的起源。最后,我们对该领域存在的挑战以及未来的前景进行了展望。
王雪 , 张丽 , 刘长鹏 , 葛君杰 , 祝建兵 , 邢巍 . 碱性介质中非贵金属氧还原催化剂的结构调控进展[J]. 电化学, 2022 , 28(2) : 2108501 . DOI: 10.13208/j.electrochem.210850
Oxygen reduction reaction (ORR) in alkaline electrolytes is an important electrochemical process for metal-air batteries and anion exchange membrane fuel cells (AEMFCs). However, the sluggish kinetics spurs intensive research on searching robust electrocatalysts. Non-precious metal catalysts (NPMCs) that can circumvent the cost and scarcity issues associated with platinum (Pt)-based materials have been pursued and the challenges lie in the performance improvement to rival Pt-based benchmarks. As the composition and structure of the NPMCs have a significant impact on the catalytic performance, precise regulation on the catalyst structure holds great promise to bridge the activity gap between NPMCs and Pt-based benchmarks. In this minireview, we aim to provide an overview of recent progress in the structural regulation on NPMCs towards improved performance. The four typical categories of NPMCs, i.e., metal-free carbon-based materials, metal compounds, metal encapsulated in graphitic layer and atomically dispersed metal-nitrogen-carbon materials, are firstly introduced, where catalytic active sites and catalytic mechanism are highlighted. Subsequently, we summarize the representative structural regulation from a nanoscale to an atomic scale including hierarchically porous structure regulation, interface engineering, defect engineering and atomic pair construction. Special emphasis is placed on the elucidation of the catalytic structure-performance relationship. The origins of activity improvements from these structural regulations are discussed in terms of accelerated mass transfer, increased accessible active sites, tailored electronic states, and synergetic effect between multi-components. Finally, the challenges and opportunities are discussed.

/
| 〈 |
|
〉 |