

磷化物助力铂基催化剂甲醇电氧化的研究进展
收稿日期: 2021-06-21
修回日期: 2021-08-03
网络出版日期: 2021-08-05
版权
Advances of Phosphide Promoter Assisted Pt Based Catalyst for Electrooxidation of Methanol
Received date: 2021-06-21
Revised date: 2021-08-03
Online published: 2021-08-05
Copyright
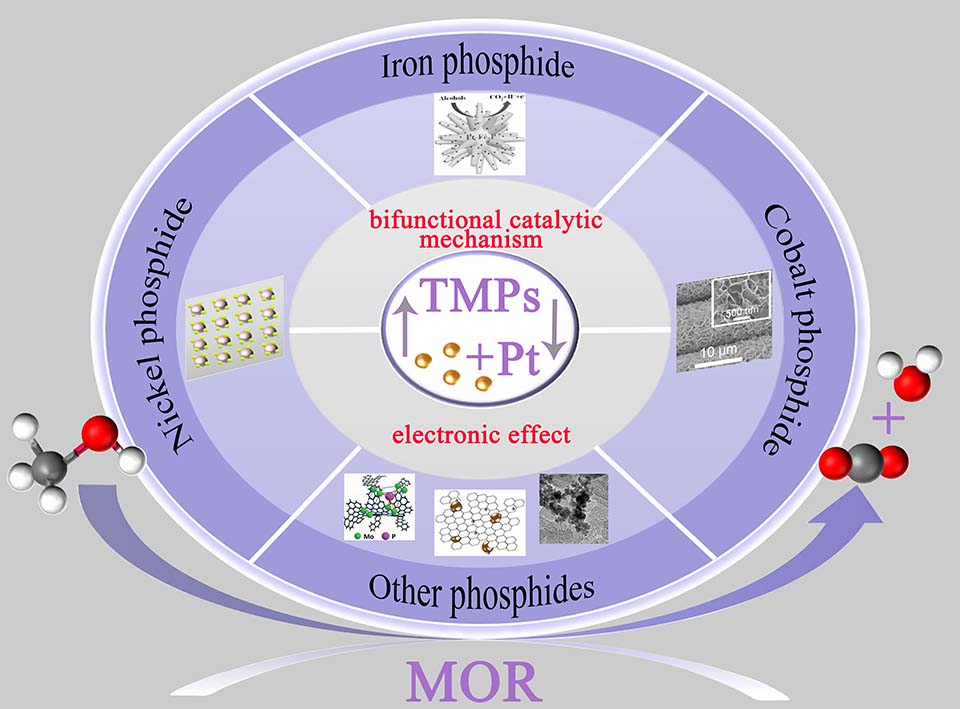
过渡金属磷化物(TMP)作为一种理想的甲醇电氧化助催化剂,因其具有多功能活性位点、结构和组成可调、独特的物理化学性质和高效的多组分协同效应等优势而受到越来越多的关注。本文综述了过渡金属磷化物促进甲醇电氧化的研究进展,包括催化剂的制备及其催化甲醇电氧化的性能评估。首先,介绍了TMP对催化甲醇氧化反应的促进作用,然后在正文中讨论了基于不同金属中心的TMP催化剂体系的制备与性能研究。从电子效应和基于双功能催化机制的亲氧性来看,TMPs对催化甲醇氧化有明显的促进作用。最后,我们讨论了在催化剂理性设计及其催化机理探索和燃料电池装置应用中应注意的问题和挑战,希望对新型催化剂体系的设计和制备有一定的指导意义。
李萌 , 冯立纲 . 磷化物助力铂基催化剂甲醇电氧化的研究进展[J]. 电化学, 2022 , 28(1) : 2106211 . DOI: 10.13208/j.electrochem.210621
Transition metal phosphide (TMP), as an ideal catalytic promoter in methanol fuel oxidation, has received increased attention because of its multifunctional active sites, tunable structure and composition, as well as unique physical and chemical properties and efficient multi-composition synergistic effect. Some advances have been made for this catalyst system recently. In the current review, the research progresses of transition metal phosphides (TMPs) in the assisted electrooxidation of methanol including the catalysts fabrication and their performance evaluation for methanol oxidation are reviewed. The promotion effect of TMPs has been firstly presented and the catalyst systems based on the different metal centers of TMPs are then mainly discussed. It is concluded that the TMPs can greatly promote methanol oxidation through the electronic effect and the oxyphilic property based on the bifunctional catalytic mechanism. The problems and challenges in methanol fuel oxidation by using TMPs are also described at the end with the attention being paid to the precise catalyst design. The catalytic mechanism probing and application of the fuel cells device are proposed. The current effort might be helpful to the community for novel catalyst system design and fabrication.

/
| 〈 |
|
〉 |