

一种用于电还原CO2生成甲酸的高性能连续流动式MEA反应器
收稿日期: 2021-04-23
修回日期: 2021-06-06
网络出版日期: 2021-06-10
版权
A High-Performance Continuous-Flow MEA Reactor for Electroreduction CO2 to Formate
Received date: 2021-04-23
Revised date: 2021-06-06
Online published: 2021-06-10
Copyright
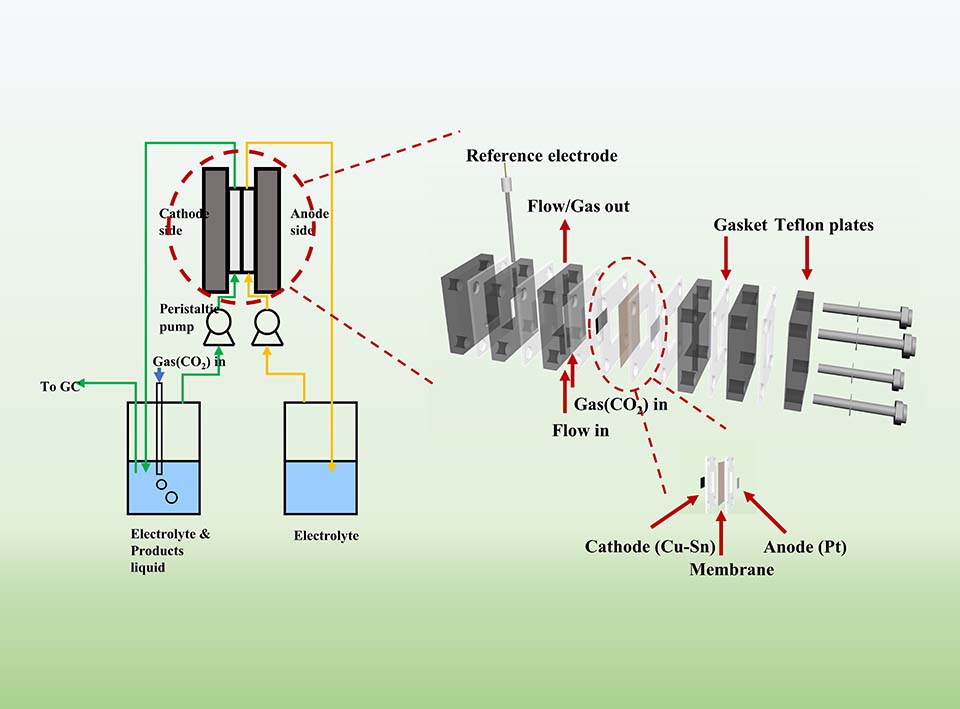
电化学二氧化碳还原反应(CO2RR)是一种利用间歇性可再生电力缓解环境问题,并且生产液体燃料和工业化学品的有前途的方法。然而,传统的H型反应器由于在电解液中较低的CO2溶解度以及两电极之间较大的极距而导致高欧姆电阻,严重限制了CO2RR的电化学性能,不利于CO2RR在工业应用的发展。在本文中,我们设计了一种基于0.5 mol·L-1 KHCO3的自生长Cu/Sn双金属电催化剂的高性能连续流膜电极组件(MEA)反应器,用于将CO2转化为甲酸。与H型反应器相比,流动式MEA反应器不仅显示出优异的电流密度(-1.11 VRHE时电流密度为66.41 mA·cm-2),而且还保持了较高的甲酸法拉第效率(89.56%),并且能够稳定工作至少20 h。本文还设计了一套新型CO2RR系统,可以有效地分离气态/液态产物。出乎意料的是,在-0.91 VRHE且电池电压为3.17 V时,甲酸的生产率为163 μmol·h-1·cm-2。本文为克服电化学CO2RR的传质限制以及分离液体和气体产物提供了一条新途径。
刘佩璇 , 彭芦苇 , 何瑞楠 , 李露露 , 乔锦丽 . 一种用于电还原CO2生成甲酸的高性能连续流动式MEA反应器[J]. 电化学, 2022 , 28(1) : 2104231 . DOI: 10.13208/j.electrochem.210423
The electrochemical carbon dioxide reduction reaction (CO2RR) is a promising approach to produce liquid fuels and industrial chemicals by utilizing intermittent renewable electricity for mitigating environmental problems. However, the traditional H-type reactor seriously limits the electrochemical performance of CO2RR due to the low CO2 solubility in electrolyte, and high ohmic resistance caused by the large distance between two electrodes, which is unbeneficial for industrial application. Herein, we demonstrated a high-performance continuous flow membranes electrode assembly (MEA) reactor based on a self-growing Cu/Sn bimetallic electrocatalyst in 0.5 mol·L-1 KHCO3 for converting CO2 to formate. Compared with an H-type cell, the MEA reactor not only shows the excellent current density (66.41 mA·cm-2 at -1.11 VRHE), but also maintains high Faraday efficiency of formate (89.56%) with the steady work around 20 h. Notably, we also designed the new CO2RR system to effectively separate the gaseous/liquid production. Surprisingly, the production rate of formate reached 163 μmol·h-1·cm-2 at -0.91 VRHE with the cell voltage of 3.17 V. This study provides a promising path to overcome mass transport limitations of the electrochemical CO2RR and to separate liquid from gas products.

Key words: electrochemical reduction; carbon dioxide; flow MEA reactor; electrolyzer
/
| 〈 |
|
〉 |