

MoS2/GQDs催化剂的制备及微生物电解池的产氢性能研究
收稿日期: 2020-07-24
修回日期: 2020-12-10
网络出版日期: 2021-01-29
基金资助
山西省高等学校科技创新计划项目(2019L1006);国家自然科学基金项目(51703151)
Preparation and Electrochemical Evaluation of MoS2/Graphene Quantum Dots as a Catalyst for Hydrogen Evolution in Microbial Electrolysis Cell
Received date: 2020-07-24
Revised date: 2020-12-10
Online published: 2021-01-29
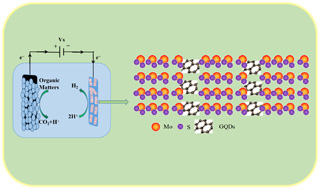
通过水热法合成了一系列MoS2/GQDs复合材料,并制成碳基复合电极。利用电化学测试手段挑选出最佳电极后用于微生物电解池(MEC)阴极的产氢性能研究。实验结果显示: Na2MoO4、半胱氨酸和GQDs的最佳原料配比为375:600:1,制备出的MoS2/GQDs呈现明显的爆米花样纳米片结构,片层厚度在10 nm左右,当碳纸负载量为1.5 mg·cm-2时,MoS2/GQDs碳纸电极的析氢催化能力最佳。在MEC产氢实验中,MoS2/GQDs阴极MEC的产气量、氢气产率、库仑效率、整体氢气回收率、阴极氢气回收率、电能回收率和整体能量回收率分别为51.15±3.15 mL·cycle-1、0.401±0.032 m3H2·m3d-1、91.16±0.054%、66.64±5.39%、72.44±2.60%、217.26±7.42%和77.37±1.50%,均略高于Pt/C阴极MEC或与之媲美。另外,MoS2/GQDs具有良好的长期稳定性,且价格便宜,有利于实际应用。
代红艳 , 杨慧敏 , 刘宪 , 宋秀丽 , 梁镇海 . MoS2/GQDs催化剂的制备及微生物电解池的产氢性能研究[J]. 电化学, 2021 , 27(4) : 429 -438 . DOI: 10.13208/j.electrochem.200725
Microbial electrolysis cell (MEC) is a relatively new bioelectrochemical technology that produces H2 and meanwhile treats organic wastewater. Cathode hydrogen evolution catalyst plays a key role in MEC. The doping of Graphene Quantum Dots (GQDs) into MoS2 nanosheets can improve the catalytic activity of MoS2 by creating abundant defect sites both in the edge plane and the basal plane, as well as enhancing the electrical conductivity. In this paper, using Na2MoO4 , cysteine and GQDs as raw materials, a series of MoS2/GQDs composites were firstly synthesized via hydrothermal method, and then loaded on the carbon-based electrode. The optimal electrode was selected by electrochemical testing methods and used as a cathode of MEC to research the hydrogen production capacity. SEM image showed that the MoS2/GQDs composite exhibited a popcorn-like nanosheet structure and the thickness of each nanosheet was about 10 nm. The specific surface area of MoS2/GQDS composite reached 67.155 m2·g-1, which was 16 times of the specific surface area of MoS2 (4.197 m2·g-1). TEM image showed that some lattice fringes representing GQDs were intermixed with lattice fringes representing MoS2, which indicated that GQDs were well embedded in MoS2 . EDS results showed that the MoS2/GQDS composite contained Mo, S, C and O, and the atomic ratio of Mo: S: C: O = 1:2.5:1.9:1.2, indicating that the majority of Mo and S in the composite existed in the form of MoS2, while a part of S existed in the form of SOx. The LSV data of MoS2/GQDs carbon paper electrode showed that the synthesized MoS2/GQDs composite (2#) had the best catalytic activity toward hydrogen evolution when the raw material ratio of Na2MoO4, cysteine and GQDs was 375:600:1 with the optimum load of 1.5 mg·cm-2. The Tafel slope of MoS2/GQDs electrode (2#, 1.5 mg·cm-2) was found to be 44.3 mV·dec-1, lower than that of pure MoS2 electrode, which indicated that the doping of GQDs into MoS2 nanosheets made the electron transport more efficiently and the interfacial resistance was significantly reduced. In the MEC tests, the maximum hydrogen current density of MoS2/GQDS cathode (2#, 1.5 mg·cm-2) MEC reached 14.70 ± 0.80 A·m-2, which was comparable to that of Pt/C cathode MEC (14.58 ± 0.92 A·m-2), indicating that MoS2/GQDS had a good catalytic activity for hydrogen evolution. The gas production, hydrogen production rate, coulombic efficiency, hydrogen recovery efficiency, cathodic hydrogen recovery efficiency, electrical and overall energy recovery efficiencies of MoS2/GQDs cathode (2#, 1.5 mg·cm-2) MEC were, respectively, 51.15±3.15 mL·cycle-1, 0.40±0.032 m3H2·m-3d-1, 91.16±0.054%, 66.64±5.39%, 72.44±2.60%, 217.26±7.42% and 77.37±1.50%, which were slightly higher than or comparable to those of Pt/C cathode MEC. In addition, MoS2/GQDs enjoyed good stability and price advantage, which might promote the practical application of MECs.

/
| 〈 |
|
〉 |