

对称季铵碱的烷基链长度对草酸电还原反应的影响
收稿日期: 2020-09-05
修回日期: 2020-10-30
网络出版日期: 2020-11-12
Effect of Alkyl Chain Length of Symmetrical Quaternary Ammonium Hydroxide on Oxalic Acid Electroreduction Reaction
Received date: 2020-09-05
Revised date: 2020-10-30
Online published: 2020-11-12
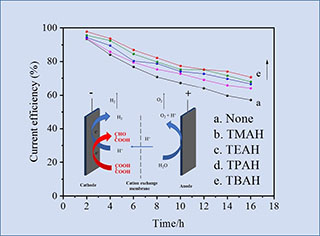
研究了四种不同烷基链长度的对称季铵碱对草酸电还原制备乙醛酸反应的影响。线性扫描测试考察了添加剂对铅电极上阴极反应的影响,结果表明对称季铵碱在电极表面的吸附对析氢反应的抑制程度大于其对草酸电还原反应的抑制程度,且随着对称季铵碱中烷基链长度的增加,添加剂抑制析氢反应效果更明显。计时安培法的结果证明添加剂可影响草酸向电极表面的扩散,随着对称季铵碱中烷基链长度的增加,草酸的扩散系数呈现出先增加后减小的趋势。恒流电解实验结果表明,添加剂能有效提高草酸电还原反应的电流效率,且提高效果随对称季铵碱所含烷基链长度的增加而增强。因此,添加剂的吸附对阴极表面析氢反应的抑制作用是草酸电还原反应电流效率提高的主要原因。本研究表明,四丁基氢氧化铵为添加剂时,草酸还原为乙醛酸的电流效率最高。
黄波 , 张新胜 , 钮东方 , 胡硕真 . 对称季铵碱的烷基链长度对草酸电还原反应的影响[J]. 电化学, 2021 , 27(5) : 529 -539 . DOI: 10.13208/j.electrochem.200830
Glyoxylic acid with the dual characteristics of acid and aldehyde is an important chemical raw material and organic synthesis intermediate, which is extensively used in the perfumery, pharmaceutical and fine chemical industries. A family of symmetric quaternary ammonium hydroxides (QAHs) with different alkyl chain lengths was used as the additives in generating glyoxylic acid from oxalic acid electroreduction reaction (OAER). The effects of alkyl chain length on OAER and the corresponding side reaction, i.e., hydrogen evolution reaction (HER), were investigated. Linear sweep voltammetric (LSV) results showed that the adsorption of the additives suppressed more on the HER than that on OAER, resulted in improving the current efficiency of OAER. As the alkyl chain length of QAH increased, the suppression effect on HER was more obvious. The effect of additives on oxalic acid diffusion was studied by chronoamperometry (CA). With the increase in the alkyl chain length of the QAH additives, the diffusion coefficient of oxalic acid increased first and then decreased. The constant-current electrolysis results showed that the additives could effectively improve the current efficiency of the OAER, which was highly related to the alkyl chain length of QAHs. The LSV, CA and electrolysis results indicate that the suppression effect of QAHs adsorption on HER is the main reason for the improvement of current efficiency. In this study, tetrabutylammonium hydroxide (TBAH) is the best additive to increase the current efficiency of generating glyoxylic acid from oxalic acid.

| [1] | Abdulwahed M, Mamoly L, Bosnali W. A simple spectrophotometric method for determination of glyoxylic acid in its synjournal mixture[J]. Int. J. Anal. Chem., 2020: 5417549. |
| [2] | Niu Y L, Xu Z, Li M, Li R F. Oxidation of glyoxal to glyoxylic acid by oxygen over V2O5/C catalyst[J]. Chin. Chem. Lett., 2008, 19(2): 245-248. |
| [3] | Hermans S, Thiltges F, Deffenez A, Devillers M. Molybdenum oxoanions as dispersing agents in the preparation of Pd/C catalysts for the selective oxidation of glyoxal[J]. Catal. Lett., 2012, 142(5): 521-530. |
| [4] | Pope F D, Gallimore P J, Fuller S J. Ozonolysis of maleic acid aerosols: Effect upon aerosol hygroscopicity, phase and mass[J]. Environ. Sci. Technol., 2010, 44(17): 6656-6660. |
| [5] | Pozdniakov M A, Zhuk I V, Lyapunova M V, Salikov A S, Botvin V V, Filimoshkin A G. Glyoxylic acid synjournal, isolation, and crystallization[J]. Russ. Chem. Bull., 2019, 68(3): 472-479. |
| [6] | Pierre G, Ziade A. The oxidation of glyoxal and ethylene glycol on platinum containing in aqueous acid mediums some metal salts[J]. Electrochim. Acta, 1987, 32(4): 601-606. |
| [7] | Kimura M, Kobayashi K, Yamamoto Y, Sawaki Y. Electrooxidative pinacol-type rearrangement of β-hydroxy sulfides. Efficient C-S cleavage mediated by chloride ion oxidation[J]. Tetrahedron, 1996, 52(12): 4303-4310. |
| [8] | Danly D E. Adiponitrile via improved EHD[J]. Hydrocarb Process, 1981, 60(4): 161-164. |
| [9] | Scott K. Electrolytic reduction of oxalic acid to glyoxylic acid: A problem of electrode deactivation[J]. Chem. Eng. Res. Des., 1986, 64(4): 266-271. |
| [10] | Ochoa J R, Diego A D, Santa-Olalla J. Electrosynjournal of glyoxylic acid using a continuously electrogenerated lead cathod[J]. J. Appl. Electrochem., 1993, 23(9): 905-909. |
| [11] | Chen B A, Xu J, Wang L M, Song L F, Wu S Y. Synjournal of quaternary ammonium salts based on diketopyrrolopyrroles skeletons and their applications in copper electroplating[J]. ACS Appl. Mater. Inter., 2017, 9(8): 7793-7803. |
| [12] | Xu J, Chen B, Lv J, Chang D D, Niu D F, Hu S Z, Zhang X S, Xin Z, Wang L M. Aryl modification of diketopyrrolopyrrole-based quaternary ammonium salts and their applications in copper electrodeposition[J]. Dyes Pigments, 2019, 170: 107559. |
| [13] | Miao Z W, Pei F B, Liu Z W, Zhang Z, Yu R J, Liu R S. Preparation of highly purity tetrabutyl ammonium hydroxide using a novel method of electro-electrodialysis: The study on mass transfer process and influencing factors[J]. J. Membrane. Sci., 2018, 567: 281-289. |
| [14] | Huang X, Tan L Q, Zhang L, Li C P, Wei Z D. Coverage-dependent acrylonitrile adsorption and electrochemical reduction kinetics on Pb electrode[J]. Chem. Eng. J., 2020, 382: 123006. |
| [15] | Blanco D E, Dookhith A Z, Modestino M A. Enhancing selectivity and efficiency in the electrochemical synjournal of adiponitrile[J]. React. Chem. Eng., 2019, 4(1): 8-16. |
| [16] | Goodridge F, Lister K, Plimley R E. Scale-up studies of the electrolytic reduction of oxalic to glyoxylic acid[J]. J. Appl. Electrochem., 1980, 10(1): 55-60. |
| [17] | Zhou Y L, Zhang X S, Dai Y C, Yuan W K. Studies on chemical activators for electrode I: Electrochemical activation of deactivating cathode for oxalic acid reduction[J]. Chem. Eng. Sci., 2003, 58(3-6): 1021-1027. |
| [18] | Jin L(金玲), Zhang X S(张新胜). Additives structure in electroreduction of oxalic acid[J]. CIESC Journal(化工学报), 2010, 61(S1): 86-90. |
| [19] | Jin L, Pang C X, Zhang X S, Niu L, Yuan W K. Determination of glyoxylic acid in organic electrosynjournal using the differential pulse polarography[J]. Asian J. Chem., 2013, 25(18): 10102-10106. |
| [20] | Wade.R C, Guilbault L J. Electrolytic method for producing quaternary ammonium hydroxides: American, US4394226-A1[P]. 1983-7-19. |
| [21] | Yang J(杨娇), Zhang X S(张新胜). Preparation of electronic tetrabutylammonium hydroxide by ion-exchange membrane electrolysis[J]. CIESC Journal(化工学报), 2010, 61(S1): 77-81. |
| [22] | Campbell C R, Spiegelhalter R R. Preparation of quaternary ammonium hydroxides by electrolysis: American, US43943265-A1[P]. 1968-9-17. |
| [23] | Scott K. The role of remperatre in oxalic acid electroreduction[J]. Electrochim. Acta, 1992, 37(8): 1381-1388. |
| [24] | Liu X(刘欣), Li Z Y(李宇展), Hu R S(胡瑞省), Gu D P(顾登平). Studies on the mechanism of electroreduction of oxalic acid[J]. J. Electrochem.(电化学), 2004, 10(1): 41-45. |
| [25] | Pickett D J, Yap K S. A study of the production of glyoxylic acid by the electrochemical reduction of oxalic acid solutions[J]. J. Appl. Electrochem., 1974, 4: 17-23. |
| [26] | Fan Y H, Haseltine J. Interactive delocalizations that control an aqueous organic equilibrium[J]. Tetrahedron Lett., 1996, 37(52): 9279-9282. |
| [27] | Liu N N, Senthil R A, Zhang X, Pan J Q, Sun Y Z, Liu X G. A green and cost-effective process for recovery of high purity α-PbO from spent lead acid batteries[J]. J. Clean. Prod., 2020, 267: 122107. |
| [28] | Ijomah M N C. Electrochemical behavior of some lead alloys[J]. J. Electrochem. Soc., 1987, 134(12): 2960-2966. |
| [29] | Zhang B, Zhong J H, Li W J, Dai Z Y, Zhang B, Cheng Z M. Transformation of inert PbSO4 deposit on the negative electrode of a lead-acid battery into its active state[J]. J. Power Sources, 2010, 195(13): 4338-4343. |
| [30] | Kawasaki A, Nishihama S, Yoshizuka K. Adsorption of tetraalkyl ammonium hydroxide with mesoporous silica[J]. Sep. Sci. Technol., 2012, 47(9): 1356-1360. |
| [31] | Marcus Y. Tetraalkylammonium ions in aqueous and non-aqueous solutions[J]. J. Solution Chem., 2008, 37(8): 1071-1098. |
| [32] | Anson F C. Chronocoulometry: A convenient, rapid and reliable technique for detection and determination of adsorbed reactants[J]. J. Chem. Educ., 1983, 60(4): 293-296. |
| [33] | Golabi S M, Irannejad L. Preparation and electrochemical study of fisetin modified glassy carbon electrode. Application to the determination of NADH and ascorbic acid[J]. Electroanalysis, 2005, 17(11): 985-996. |
| [34] | Raoof J B, Ojani R, Rashid-Nadimi S. Preparation of polypyrrole/ferrocyanide films modified carbon paste electrode and its application on the electrocatalytic determination of ascorbic acid[J]. Electrochim. Acta, 2004, 49(2): 271-280. |
| [35] | Zhu J L, Zhou Y H, Gao C Q. Influence of surfactants on electrochemical behavior of zinc electrodes in alkaline solution[J]. J. Power Sources, 1998, 72: 231-235. |
| [36] | Seo D W, Sarker S, Nath N C D, Choi S W, Ahammad A J S, Lee J J, Kim W G. Synjournal of a novel imidazolium-based electrolytes and application for dye-sensitized solar cells[J]. Electrochim. Acta, 2010, 55(4): 1483-1488. |
| [37] | Dehmlow E V. Phase-transfer catalyzed two-phase reactions in preparative organic chemistry[J]. Angew. Chem. Int. Ed., 1974, 13(3): 170-179. |
| [38] | Zhao C T(赵崇涛), Zhu Z S(朱则善). Study on synthesizing of 2-methylbutanoic acid by indirect electrooxidation[J]. J. Electrochem.(电化学), 1999, 5(3): 310-313. |
| [39] | Shabestary N, Khazaeli S, Hickman R. Phase-transfer catalytic reaction: A physical chemistry laboratory experiment[J]. J. Chem. Educ., 1998, 75(11): 1470-1472. |
| [40] | Makosza M, Fedorynski M. Phase transfer catalysis - basic principles, mechanism and specific features[J]. Curr. Catal., 2012, 1(2): 79-87. |
| [41] | Davies. J A. Synthetic coordination chemistry: Principles and practice[M]. Ohio: World Science Publishing Co. Ptc. Ltd., 1996: 362. |
| [42] | Chen W C, Ho B H. Diffusion coefficients of acrylic mono-mers in poly(methyl methylacrylate)[J]. J. Polym. Res., 1998, 5(3): 187-191. |
/
| 〈 |
|
〉 |