

纳米硅在含添加剂的高浓度电解液中循环特征及其表面协同成膜的研究
收稿日期: 2020-06-30
修回日期: 2020-07-21
网络出版日期: 2020-08-19
基金资助
国家重点研发计划项目(2016YFB0301305);国家重点研发计划项目(2018YFB0104400);国家自然科学基金项目(U1764255);国家自然科学基金项目(21903067)
Cycling Performance and Solid-Electrolyte-Interphase Synergic Formation of Silicon Nanoparticles in the Concentrated Electrolyte with Additives
Received date: 2020-06-30
Revised date: 2020-07-21
Online published: 2020-08-19
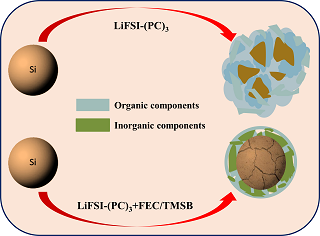
本文研究了在LiFSI-(PC)3高浓度电解液中添加剂对于纳米硅材料的循环性能的影响,采用扫描电子显微镜、傅里叶变换红外光谱和X-射线光电子能谱分析了循环过程纳米硅材料及其电极的结构和表面SEI膜演化的特征. 结果表明,添加剂能够改善纳米硅材料的循环性能,在LiFSI-(PC)3高浓度电解液中循环300周材料比容量为574.8 mAh·g-1,而含有3%LiDFOB、3%FEC、3%TMSB的添加剂的高浓度电解液中,比容量分别为1142.9、1863.6和1852.2 mAh·g-1. 作者分析认为,在LiFSI-(PC)3浓溶液中LiFSI优先于PC在纳米硅表面发生成膜反应,形成的SEI膜由以无机物主导的内层膜和以有机物主导的外层膜组成,而在含添加剂的高浓度电解液中,添加剂和LiFSI协同参与SEI成膜反应,形成的内层膜能够减缓PC溶剂参与外层的成膜反应,由此形成的SEI膜能够抑制循环过程中SEI膜的过度生长,更好地抑制了纳米硅的粉化,纳米硅材料及其电极结构稳定性更好,材料表现出更好的循环性能.
常增花 , 韩富娟 , 杨夕馨 , 王建涛 , 卢世刚 . 纳米硅在含添加剂的高浓度电解液中循环特征及其表面协同成膜的研究[J]. 电化学, 2020 , 26(5) : 759 -771 . DOI: 10.13208/j.electrochem.200645
In this paper, the effects of additives on the cycling performance of silicon nanoparticles in LiFSI-(PC)3 based concentrated electrolytes were systematically studied. The structures of silicon nanoparticle electrodes and the evolution of solid-electrolyte-interphase were characterized by scanning electron microscopy (SEM), attenuated total reflection Flourier transformed infrared spectroscopy (ATR-FTIR) and X-ray photoelectron spectroscopy (XPS). The results indicated that the additives can efficiently improve the cycling performance of silicon nanoparticle electrodes. In LiFSI-(PC)3 concentrated electrolyte, the capacity became 574.8 mAh·g-1 after 300 cycles with the initial capacity of 3296.1 mAh·g-1. In contrast, the 3% LiDFOB, 3% FEC and 3% TMSB-containing systems reached 1142.9, 1863.6 and 1852.2 mAh·g-1 after 300 cycles, respectively. The comprehensive analysis indicates that the reduction of LiFSI takes priority over PC on the surface of silicon nanoparticles in LiFSI-(PC)3 concentrated electrolyte, and the SEI film is composed of an inner layer dominated by inorganic products and an outer layer dominated by organic products. While in the concentrated electrolyte containing additives, the additives and LiFSI participate in the formation of SEI inner layer synergistically, and the SEI inner layer can suppress the reduction of PC which contribute to the formation of SEI outer layer. The SEI film formed on this mechanism could suppress the excessive growth of the SEI film, mitigate the pulverization of silicon nanoparticles, and enhance the structure stability of the silicon nanoparticle electrode, thus, the silicon nanoparticle electrodes exhibited better cycling performance.

/
| 〈 |
|
〉 |