

PdCoIr四面体合金纳米催化剂的制备及其对乙醇氧化的电催化性能
收稿日期: 2020-05-15
修回日期: 2020-06-09
网络出版日期: 2020-06-10
基金资助
国家自然科学基金项目(21802112);国家自然科学基金项目(21773198)
Preparation of PdCoIr Tetrahedron Nanocatalysts and Its Performance toward Ethanol Oxidation Reaction
Received date: 2020-05-15
Revised date: 2020-06-09
Online published: 2020-06-10
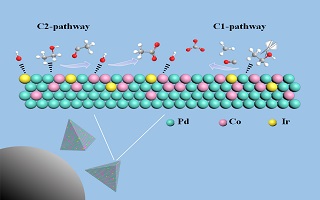
钯(Pd)基催化剂是直接乙醇燃料电池研究中广泛使用的催化剂,进一步提升其性能是推动燃料电池发展的重要方向。本文用一步水热法制备出四面体结构PdCo(PdCo tetrahedron,记为PdCo-TH)和少量铱(Ir)掺杂的PdCo四面体合金纳米粒子(记为PdCoIr-TH)。经TEM、ICP、XPS及CV等表征证实,PdCoIr-TH为三元合金纳米粒子,且掺杂的Ir元素倾向分布在催化剂表层。相比于商业Pd/C催化剂,PdCo-TH/C和PdCoIr-TH/C对乙醇电氧化的催化性能显著增强。研究结果表明,Pd9Co1Ir0.1-TH/C在低电位(< -0.25 V)下具有最高的乙醇电氧化活性和稳定性。Ir掺杂不仅提高了催化剂抗CO毒化的能力还有利于乙醇起始氧化电位负移。同时,随着Ir含量的增加,所制备的纳米催化剂的乙醇电氧化C1产物选择性也随之升高。针对不同组成催化剂反应性的差异,本文认为Co与Ir位点上容易产生OHad物种,这将有利于活性Pd位点上乙醇电氧化中间反应物种的有效转化。除了以上的各位点间的协同效应,三元合金的形成,进一步调控了Pd的d带电子结构,从而促进了催化剂反应性的改变。
余志远 , 黄蕊 , 刘杰 , 李广 , 宋前通 , 孙世刚 . PdCoIr四面体合金纳米催化剂的制备及其对乙醇氧化的电催化性能[J]. 电化学, 2021 , 27(1) : 63 -75 . DOI: 10.13208/j.electrochem.200515
As a new energy conversion device, direct ethanol fuel cells (DEFCs) are widely concerned because of their remarkable advantages such as high theoretical energy density and wide fuel sources. However, the rapid development of DEFCs has been severely impeded due to the sluggish kinetic process and toxic intermediates especially in their anodic reactions. Palladium (Pd)-based materials are considered to be excellent anode catalysts for DEFCs, especially under alkaline conditions. And further improving their performance is an important direction to promote the development of DEFCs. Surface structure and composition are the key factors affecting the performance of catalysts which can be improved by reasonable regulation. It is reported that high-index faceted structures and element doping are beneficial to improve the performance of catalyst. In this work, the advantages of these two strategies were used comprehensively to prepare Pd-based catalysts with high efficiency. Palladium cobalt (PdCo) and Ir-doped PdCo tetrahedron alloy nanocatalysts (denoted by PdCo-TH and PdCoIr-TH, respectively) have been successfully prepared by one-step hydrothermal method. The characterization results of TEM, ICP, XPS and CV show that the PdCo-TH binary and PdCoIr-TH ternary alloys were formed, while Ir element was mainly distributed on the PdCoIr-TH surface. Compared with the commercial Pd/C, the PdCo-TH/C and PdCoIr-TH/C exhibited the enhanced catalytic properties toward ethanol oxidation reaction in alkaline solutions. Particularly, the Pd9Co1Ir0.1-TH/C catalyst showed the best activity and stability toward EOR, especially at low potentials (< -0.25 V). And Ir sites not only resisted CO poison effectively, but also shifted the initial oxidation potential of ethanol negatively. Meanwhile, the selectivity of C1 products during the electrocatalytic oxidation of ethanol has been greatly improved with the increase of Ir content. The enhanced reactivities of PdCo-TH/C and PdCoIr-TH/C could be attributed to: (a) The coexistence of Co sites and Ir sites on the surfaces can generate OHad species which can promote the oxidation of intermediate adsorbed species on Pd sites and (b) the negative shift in electron binding energy of Pd due to the addition of Ir may make reaction intermediates desorb more difficultly, which might make the reactivity of PdCoIr-TH/C differ from that of PdCo-TH/C. This research work has demostrated a strategic approach for future development in high efficiency catalysts used for DEFCs.

/
| 〈 |
|
〉 |