

经由[Ni(en)3](SeO3)配合物电镀制备的硒化镍高效电催化水分解反应
收稿日期: 2019-05-07
修回日期: 2019-09-29
网络出版日期: 2019-10-28
Nickel Selenide Derived from [Ni(en)3](SeO3) Complex for Efficient Electrocatalytic Overall Water Splitting
Received date: 2019-05-07
Revised date: 2019-09-29
Online published: 2019-10-28
Supported by
We are grateful for the Starting Research Funds of Shaanxi Normal University, the National Natural Science Foundation of China (Grant Nos. 21503126, 21573139, 21773146 and 21872092).
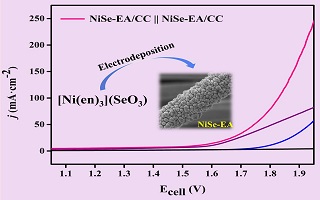
陈丹丹 , 高学庆 , 刘红飞 , 张 伟 , 曹 睿 . 经由[Ni(en)3](SeO3)配合物电镀制备的硒化镍高效电催化水分解反应[J]. 电化学, 2019 , 25(5) : 553 -561 . DOI: 10.13208/j.electrochem.181141

/
| 〈 |
|
〉 |