

暂态电化学表面增强拉曼光谱研究对硝基苯硫酚分子的电化学还原过程
收稿日期: 2019-07-09
修回日期: 2019-09-18
网络出版日期: 2019-12-28
基金资助
国家自然科学基金项目(No. 21573043, No. 21473140)、福建省自然科学基金面上项目(No. 2019J01746)以及食品安全与生物分析教育部重点实验室开放基金(No. FS18019)资助
Transient Electrochemical Surface-Enhanced Raman Spectroscopic Study in Electrochemical Reduction of P-Nitrothiophenol
Received date: 2019-07-09
Revised date: 2019-09-18
Online published: 2019-12-28
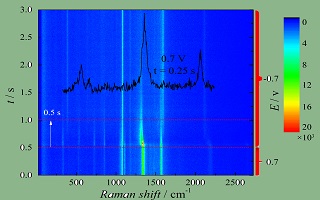
关键词: 暂态电化学-表面增强拉曼光谱; 时间分辨表面增强拉曼光谱; 循环伏安法; 计时电流法; 对硝基苯硫酚
凌 云 , 汤 儆 , 刘国坤 , 宗 铖 . 暂态电化学表面增强拉曼光谱研究对硝基苯硫酚分子的电化学还原过程[J]. 电化学, 2019 , 25(6) : 731 -739 . DOI: 10.13208/j.electrochem.190709

/
| 〈 |
|
〉 |