

高镍正极材料(LiNi0.83Co0.12Mn0.05O2)45°C循环失效机理研究
Degradation Mechanism of LiNi0.83Co0.12Mn0.05O2 Cycled at 45 oC
Received date: 2019-01-23
Revised date: 2019-03-29
Online published: 2019-04-09
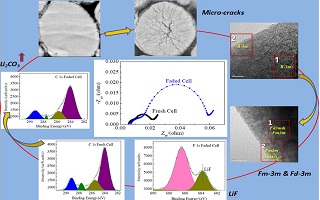
本文研究了高镍NCM811材料LiNi0.83Co0.12Mn0.05O2高温45 °C循环失效机理. 通过电化学交流阻抗谱(EIS)技术分析发现45 °C循环失效前后SEI膜阻抗(RSEI)和电荷转移阻抗(Rct)增长率最快,分别达到83.43%和211.34%. 采用XPS、TEM及FFT转换、XRD、XANES等手段分别分析了RSEI和Rct增长的主要影响因素. 其中,RSEI增长因素主要包括部分有机SEI膜组分转化成碳酸锂等无机成分,同时反应生成的LiF富集在活性物质周围,SEI膜厚度增长,阻抗升高. Rct增长因素主要包括晶体结构被破坏,层状晶相结构向尖晶石和岩盐相的转化,材料开裂,使电荷转移阻抗增加. 此外,对固相传质阻抗(Rw)影响因素也进行了分析,主要包括锂镍混排加剧,过渡金属元素溶出导致锂离子固相传质阻抗上升.
关键词: LiNi0.83Co0.12Mn0.05O2; 45 °C循环失效; 电化学阻抗谱; 固体电解质界面膜; 电荷转移阻抗
马洪运 , 姚晓辉 , 妙孟姚 , 易阳 , 伍绍中 , 周江 . 高镍正极材料(LiNi0.83Co0.12Mn0.05O2)45°C循环失效机理研究[J]. 电化学, 2020 , 26(3) : 431 -440 . DOI: 10.13208/j.electrochem.190123
Nickel-rich layered material has been considered as the most promising one for lithium ion batteries due to its high specific capacity. To further improve the lifetime performance, it is significant to investigate the degradation mechanisms deeply. In this study, the degradation mechanism of NCM811 (LiNi0.83Co0.12Mn0.05O2) being cycled at 45oC was systematically researched. Electrochemical impedance spectrum (EIS) results showed that the SEI resistance (RSEI) and charge transfer resistance (Rct) went up to 83.41% and 211.34% before and after the NCM811 material being cycled at 45oC, respectively. The main factors that influenced the RSEI and Rct were analyzed by means of XPS, TEM, XRD and XANES. The increase of RSEI was mainly ascribed to the conversion of some organic components to inorganic components such as lithium carbonate. Besides, the thickness of SEI film increased due to the side product of lithium fluoride (LiF) accumulated around the active materials. The increase of Rct was ascribed to the destruction of crystal structure, the phase transformations from R-3m to Fd-3m and to Fm-3m, and the micro-cracks appeared inside of the active particles. In addition, the solid mass transfer resistance (Rw) was found to become larger, which was mainly affected by the enhanced Li/Ni mixing and dissolutions of transition metal elements.

/
| 〈 |
|
〉 |