

具特定晶面且大比表面积贵金属纳米晶催化基元的构筑
收稿日期: 2018-09-17
修回日期: 2018-11-06
网络出版日期: 2018-11-22
基金资助
国家自然科学基金项目(No. 21333008,No. 21773190,No. 21771153,No. 21721001)、国家重大研究计划项目(No. 2015CB932301)和国家重点研发计划项目(No. 2017YFA0206801)资助
Constructions of Noble Metal Nanocrystals with Specific Crystal Facets and High Surface Area
Received date: 2018-09-17
Revised date: 2018-11-06
Online published: 2018-11-22
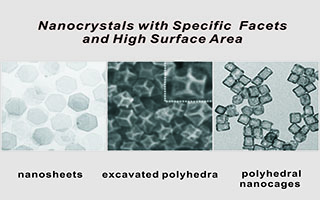
陈巧丽 , 李慧齐 , 蒋亚琪 , 谢兆雄 . 具特定晶面且大比表面积贵金属纳米晶催化基元的构筑[J]. 电化学, 2018 , 24(6) : 602 -614 . DOI: 10.13208/j.electrochem.180851

/
| 〈 |
|
〉 |