

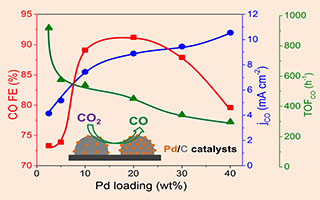
Pd/C催化剂用于CO2电化学还原生成CO:Pd载量的影响
收稿日期: 2018-09-05
修回日期: 2018-09-17
网络出版日期: 2018-09-21
Pd/C Catalysts for CO2 Electroreduction to CO:Pd Loading Effect
Received date: 2018-09-05
Revised date: 2018-09-17
Online published: 2018-09-21
Supported by
We gratefully acknowledge financial support from the Ministry of Science and Technology of China (Grant 2017YFA0700102), the National Natural Science Foundation of China (Grants 21573222 and 91545202), Outstanding Youth Talent Project of Dalian (2017RJ03), Dalian Institute of Chemical Physics (Grant DICP DMTO201702), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB17020200). G.X. Wang thanks the financial support from CAS Youth Innovation Promotion (Grant No.2015145).support from CAS Youth Innovation Promotion (Grant No. 2015145).
高敦峰 , 阎程程 , 汪国雄 , 包信和 . Pd/C催化剂用于CO2电化学还原生成CO:Pd载量的影响[J]. 电化学, 2018 , 24(6) : 757 -765 . DOI: 10.13208/j.electrochem.180845

/
| 〈 |
|
〉 |